Abstract
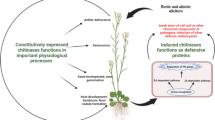
Pathogenesis-related proteins (PR-proteins) are induced in plants in response to attack by microbial or insect pests. They have been classified into several groups (PR-1 through PR-14 at present) based on their amino acid sequences and biochemical functions. Many of these proteins that have been purified from infected plants or seed extracts possess antifungal or insecticidal activity. Genes and cDNA clones for all classes of PR-proteins have been isolated from a variety of cereals. Some of these genes/cDNAs have been used to transform cereals. This review presents a summary of the PR-proteins and their genes characterized from rice, wheat, barley, sorghum and maize. Efforts to improve disease or insect resistance of these cereal plants by genetic engineering using genes for PR-proteins also are discussed. In many cases, the expression of the PR-proteins either singly or in combination appears to improve resistance to fungi or insects. In addition, chromosomal location of the PR-protein genes indicates that members of the same family of PR-protein genes or sometimes even several families of PR-protein genes often are clustered in the cereal genome, suggesting coordinate regulation. Some of these PR-protein genes map closely to quantitative traits loci. Some concerns regarding the use of genes encoding PR-proteins for genetic modification of cereals also are addressed.
Similar content being viewed by others
References
Abe K, Emori Y, Kondo H, Suzuki K & Arai S (1987) Molecular cloning of a cysteine protease inhibitor of rice (oryzacystatin): Homology with animal cystatins and transient expression in the ripening process of rice seeds. J. Biol. Chem. 262: 16793-16797
Akiyama T, Kaku H, Shibuya N (1996) Purification and properties of a basic endo1-3-β-glucanase from rice (Oryza sativa L.). Plant Cell Physiol. 37: 702-705
Akiyama T, Shibuya N, Hrmova M & Fincher GB (1997) Purification and characterization of a (163)-beta-D-glucan endohydrolase from rice (Oryza sativa) bran. Carbohydr. Res. 14: 365-374
Alexander D, Goodman RM, Gut-Rella M et al. (1993) Increased tolerance to two Oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein-1a. Proc. Natl. Acad. Sci. USA 90: 7327-7331
Altpeter, F, Diaz I, McAuslane H, Gaddour K, Coabonero P & Vasil IK (1999) Increased insect resistance in transgenic wheat stably transformed expressing trypsin inhibitor Cme. Molec. Breed. 5(1): 53-63
Anderson P-P & Pandya-Lorch R (1999) Securing and sustaining adequate world food production for the third millennium. In: World Food Security and Sustainability: The Impacts of Biotechnology and Industrial Consolidation. National Agricultural Biotechnology Council Report 11 (pp. 27-48). NABC, Ithaca, NY
Antoniw JF, Ritter CE, Pierpoint WS et al. (1980) Comparison of three pathogenesis-related proteins from plants of two cultivars of tobacco infected with TMV. J. Gen. Virol. 47: 79-87
Anuratha CS, Huang J-K, Pingali A & Muthukrishnan S (1992) Isolation and characterization of a chitinase and its cDNA clone from rice. J. Plant Biochem. Biotech. 1: 5-10
Anuratha CS, Zen KC, Cole KC, Mew T & Muthukrishnan S (1996) Induction of chitinases and β-glucanases in Rhizoctonia solani-infected rice plants: Isolation of an infection-related chitinase cDNA clone. Physiol. Plant. 97: 39-46
Asao H, Nishizawa Y, Arai S, Sato T, Hirai M, Yoshida K, Shinmyo A & Hibi T (1997) Enhanced resistance against a fungal pathogen Sphaerotheca humuli in transgenic strawberry expressing a rice chitinase gene. Plant Biotechnol. (Tokyo) 14(3): 145-149
Baga M, Chibbar RN & Kartha KK (1995) Molecular cloning and expression analysis of peroxidase genes from wheat. Plant Molec. Biol. 29: 647-662
Bartnicki-Garcia S (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Ann. Rev. Microbiol. 22: 87-108
Beffa RS & Meins F (1996) Pathogenesis-related functions of plant β-1,3-glucanases investigated by antisense transformation — A review. Gene 179: 97-103
Beffa RS, Hofer RM, Thomas M et al., (1996) Decreased susceptibility to viral disease of β-1,3-glucanase-deficient plants generated by antisense transformation. Plant Cell 8(6): 1001-1011
Bliffeld M, Mundy J, Potrykus I & Fütterer J (1999) Genetic engineering of wheat for increased resistance to powdery mildew disease. Theor. Appl. Genet. 98(6/7): 1079-1086
Bolter AJ & Jongsma MA (1995)Colorado potato beetle (Leptinotarsa decemlineata) adapt to proteinase inhibitors induced in potato leaves by methyl jasmonate. J. Insect. Physiol. 41: 1071-1078
Bown DP, Wilkinson SH & Gatehouse JA (1997) Differentially regulated inhibitor-sensitive and insensitive protease genes from the phytophagous insect pest, Helicoverpa armigera, are members of complex multigene families. Insect Biochem. Molec. Biol. 27: 625-638
Broglie K, Chet I, Holliday M et al., (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254(5035): 1194-1197
Bryngelsson T & Green B (1989) Characterization of a pathogenesis-related, thaumatin-like protein isolated from barley challenged with an incompatible race of mildew. Physiol. Molec. Plant Pathol. 35: 45-52
Bryngelsson T, Sommer-Knudsen J, Gregersen PL et al. (1994) Purification, characterization, and molecular cloning of basic PR-1-type pathogenesis-related proteins from barley. Molec. Plant-Microbe Interact. 7: 267-275
Bucciaglia PA & Smith AG (1994) Cloning and characterization of Tag1, a tobacco anther β-1,3-glucanase expressed during tetrad dissolution. Plant Molec. Biol. 24: 903-914
Chareonpornwattana S, Thara KV, Wang L, Datta SK, Panbangred W & Muthukrishnan S (1999) Inheritance, expression, and silencing of a chitinase transgene in rice. Theor. Appl. Genet. 98: 371-378
Chen MS, Johnson B, Wen L, Muthukrishnan S, Kramer KJ, Morgan TD & Reeck GR (1992) Rice cystatin: bacterial expression, purification, cysteine proteinase inhibitory activity, and insect growth suppressing activity of truncated form of the protein. Protein Expr. Purif. 3: 41-49
Chen L, Zhang S, Beachy RN & Fauquet CM (1998) A protocol for consistent, large-scale production of fertile transgenic rice plants. Plant Cell Rep. 18: 25-31
Chen WP, Gu X, Liang GH, Muthukrishnan S, Chen PD, Liu DJ & Gill BS (1998) Introduction and constitutive expression of a rice chitinase gene in bread wheat using biolistic bombardment and the bar gene as a selectable marker. Theor. Appl. Genet. 97: 1296-1306
Chen WP, Chen PD, Liu DJ, Kynast RJ, Friebe B, Velazhahan R, Muthukrishnan S & Gill BS (1999) Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor. Appl. Genet. 99: 755-760
Chibbar RN, Kartha KK, Datla RSS, Leung N, Caswell K, Mallard CS & Steinhauer L (1993) The effect of different promoter-sequences on transient expression of gus reporter gene in cultured barley (Hordeum vulgare L.) cells. Plant Cell Rep. 12: 506-509
Collinge DB & Slusarenko AJ (1987) Plant gene expression in response to pathogens. Plant Molec. Biol. 9: 389-401
Cordero MJ, Raventos D & San Segundo B (1994a) Expression of a maize protease inhibitor is induced in response to wounding and fungal infection: Systemic wound response of a monocot gene. Plant J. 6: 141-150
Cordero MJ, Raventos D & San Segundo B (1994b) Differential expression and induction of chitinases and β-1,3-glucanases in response to fungal infection during germination of maize seeds. Molec. Plant-Microbe Interact. 7: 23-31
Daniell WF (1852) On the Synsepalum dulcificum, Decand or miraculous berry of Western Africa Pharm. J. 11: 445
Datta SK & Muthukrishnan S (1999) Pathogenesis-related proteins in plants. CRC Press, Boca Raton, FL
Datta K, Tu J, Baisakh N, Oliva NP, Torrizo LB, Abrigo EM, Ona I, Mew TM & Khush GS (1999a) Genetically engineered rice for enhanced resistance to sheath blight disease. Abstract, General Meeting of the International Program on Rice Biotechnology, Sept. 20-24, 1999, Phuket, Thailand
Datta K, Velazhahan R, Oliva N, Mew T, Muthukrishnan S & Datta SK (1999b) Over-expression of thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmentally friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor. Appl. Genet. 98: 1138-1145
Datta SK, Torrizo LB, Tu J, Oliva NP & Datta K (1997) Production and molecular evaluation of transgenic plants. IRRI Discussion paper 21, International Rice Research Institute, Los Banos, Philippines
De Jong AJ, Cordewener J, Lo Schiavo F et al. (1992) A carrot somatic embryo mutant is rescued by chitinase. Plant Cell 4: 425-433
De Jong AJ, Heidstra R, Spaink HP et al. (1993)Rhizobium lipooligosaccharides rescue a carrot somatic embryo mutant. Plant Cell 5(6): 615-620
Duan X, Li X, Xue Q, Abo El-Saad M, Xu D & Wu R (1996) Transgenic rice plants expressing a proteinase inhibitor II gene are insect resistant. Nature Biotechnol. 14: 494-498
Faris JD, LiWL, Liu DJ, Chen PD & Gill BS (1999) Candidate gene analysis of quantitative disease resistance in wheat. Theor. Appl. Genet. 98: 219-225
Ghareyazie B, Menguito C, Rubia LG, De Palma J, Ona A, Muthukrishnan S, Velazhahan R, Khush & Bennett J (2000) Insect resistant transgenic aromatic rice is expressing a barley chitinase (CHI) gene and is resistant against sheath blight. Proceedings of International Rice Research Conference 2000, March 31 to April 3, 2000. International Rice Research Institute, Los Banos, Philippines
Gianinazzi S & Vallée JC (1969) Température et synthèse de matériel protéique viral chez le Nicotiana xanthi n.c. infecté par le virus de la mosaïque du tabac. C.R. Acad. Sci. Paris 269D: 593-595
Glandorf DC M, Bakker PAH & Van Loon LC (1997) Influence of the production of antibacterial and antifungal proteins by transgenic plants on the saprophytic soil microflora. Acta Bot. Neerl. 46(1): 85-104
Godwin I & Chickwamba R (1994) Transgenic grain sorghum (Sorghum bicolor) plants via Agrobacterium. In: Henry RJ & Ronalds JA (eds) Improvement of Cereal Quality by Genetic Engineering (pp. 47-53). Plenum Press, New York
Green TR & Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science 175: 776-780
Ham KS, Kauffmann S, Albersheim P et al. (1991) Host-pathogen interactions XXXIX. A soybean pathogenesis-related protein with β-1,3-glucanase activity releases phytoalexin elicitor-active heat-stable fragments from fungal walls. Mol. Plant-Microbe Interact. 4(6): 545-552
Hejgaard J, Jacobsen S & Svendsen I (1991) Two antifungal thaumatin-like proteins from barley grain. FEBS Lett. 291: 127-131
Hejgaard J, Jacobsen S, Bjorn SE & Kragh LM (1992) Anti-fungal activity of chitin-binding PR-4 type proteins from barley grain and stressed leaf. FEBS Lett. 307: 389-392
Hrmova M & Fincher GB (1993) Purification and properties of three (1,3)-β-D-glucanase isoenzymes from young leaves of barley (Hordeum vulgare). Biochem J. 289: 453-461
Huang JK, Wen L, Swegle M, Tran HC, Thin TH, Naylor HM, Muthukrishnan S & Reeck GR (1991) Nucleotide sequence of a rice genomic clone that encodes a class I endochitinase. Plant Molec. Biol. 16: 479-480
Huynh QK, Hironaka CM, Levine EB et al. (1992a) Antifungal proteins from plants — Purification, molecular cloning, and antifungal properties of chitinases from maize seed. J. Biol. Chem. 267(10): 6635-6640
Huynh QK, Borgemeyer JR & Zobel JF (1992b) Isolation and characterization of a 22 kDa protein with antifungal properties from maize seeds. Biochem. Biophys. Res. Commun. 182: 1-5
Ignatius MJS, Huang J-K, Chopra RK & Muthukrishnan S (1994a) Isolation and characterization of a barley chitinase genomic clone-Expression in powdery-mildew infected barley.J Plant Bioch. Biotech. 3: 91-95
Ignatius MJS, Chopra RK & Muthukrishnan S (1994b) Effects of fungal infection and wounding on the expression of chitinases and β-1,3-glucanases in near-isogenic lines of barley. Physiol. Plant. 90: 584-592
Inui H, Yamaguchi Y, Ishigami Y, Kawaguchi S, Yamada T, Ihara H & Hirano S (1996) Three extracellular chitinases in suspensioncultured rice cells elicited by N-acetylchitooligosaccharides. Biosci. Biotechnol. Biochem. 60: 1956-1961
Irie K, Hosoyama H, Takeuchi T, Iwabuchi K, Watanabe H, Abe M, Abe K, Arai S (1996) Transgenic rice established to express corn cystatin exhibits strong inhibitory activity against insect gut proteinases. Plant Molec. Biol. 30: 149-157
Izhar S & Frankel R (1971) Mechanism of male sterility in Petunia: The relationship between pH, callase activity in the anthers, and the breakdown of the microsporogenesis. Theor. Appl. Genet. 41: 104-108
Jacobsen S, Millelsen JD & Hejgaard J (1990) Characterization of two antifungal endochitinases from barley grain. Physiol. Plant. 79: 554-562
Jain RK, Jain S, Wang B & Wu R (1996) Optimization of biolistic method for transient gene expression and production of agronomically useful transgenic Basmati rice plants. Plant Cell Reports 15: 963-968
Jensen LG, Politz O, Olsen O, Thomsen KK & Wettstein DV (1998) Inheritance of a codon-optimized transgene expressing heat stable (1,3-1,4)-β-glucanase in scutellum and aleurone of germinating barley. Hereditas 129: 215-225
Jin W, Horner HT & Palmer RG (1997) Genetics and cytology of a new genic male-sterile soybean [Glycine max (L.) Merr.]. Sex Plant Reprod. 10(1): 13-21
Johansson T & Nyman PO (1996) A cluster of genes encoding major isozymes of lignin peroxidase and manganese peroxidase from the white-rot fungus Trametes versicolor. Gene 170: 31-38
Jongedijk E, Tigelaar H, Van Roekel JSC et al. (1995) Synergistic activity of chitinases and β-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85: 173-180
Jutidamrongphan W, Andersen JB, Mackinnon G, Manners JM, Simpson RS & Scott KJ (1991) Induction of β-1,3-glucanase in barley in response to infection by fungal pathogens. Molec. Plant Microbe Interact. 4: 234-238
Kauffmann S, Legrand M, Geoffroy P et al. (1987) Biological function of ‘pathogenesis-related’ proteins: Four PR proteins of tobacco have 1,3-β-glucanase activity. EMBO J. 6: 3209-3212
Kohli A, Leech M, Vain P, Laurie DA, Christou P (1998) Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot spots. Proc. Natl. Acad. Sci. USA 95: 7203-7208
Kohli A, Griffiths S, Palacios N, Twyman RM, Vain P, Laurie DA, Christou P (1999) Molecular characterization of transforming plasmid rearrangements in transgenic rice reveals a recombination hot spot in the CaMV 35S promoter and confirms the predominance of microhomology mediated recombination. Plant J. 6: 591-601
Kragh KM, Jacobsen S, Mikkelsen JD & Nielsen KA (1991) Purification and characterization of three chitinases and one β-1,3-glucanase accumulating in the medium of cell suspension cultures of barley (Hordeum vulgare L.). Plant Sci. 76: 65-77
Kragh KM, Jacobsen S, Mikkelsen JD & Nielsen KA (1993) Tissue specificity and induction of class I, II and III chitinases in barley (Hordeum vulgare) Physiol. Plant. 89: 490-498
Kragh KM, Hendriks T, De Jong AJ et al. (1996) Characterization of chitinases able to rescue somatic embryos of the temperature-sensitive carrot variant ts11. Plant Molec. Biol. 31: 631-645
Krishnaveni S, Liang GH, Muthukrishnan S & Manickam A (1999a) Purification and partial characterization of chitinases from sorghum seeds. Plant Sci. 144: 1-7
Krishnaveni S, Muthukrishnan S, Liang GH, Wilde G & Manickam A (1999b) Induction of chitinases and β-1,3-glucanases in resistant and sensitive cultivars of sorghum in response to insect attack, fungal infection and wounding. Plant Sci. 144: 9-16
Kung, SD (1993) Introduction. In: Kung SD & Wu R (eds) From Green Revolution to Gene Revolution 2 (pp. xvii-xxxi). Academic Press, New York
Kuranda MJ & Robbins PW (1991) Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266: 19758-19767
Kuwabara C, Arakawa K & Yoshida S (1999) Abscisic acid-induced secretory proteins in suspension-cultures cells of winter wheat. Plant Cell Physiol. 40: 184-191
Lai DM, Hoj PB & Fincher GB (1993) Purification and characterization of (1,3-1,4)-beta-glucan endohydrolases from germinated wheat (Triticum aestivum). Plant Molec. Biol. 22: 847-859
Leah R, Tommerup H, Mundy J & Svendsen I (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J. Biol. Chem. 266(3): 1564-1573
Legrand M, Kauffmann S, Geoffroy P et al. (1987) Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc. Natl. Acad. Sci. USA 84: 6750-6754
Li CD, Langridge P, Lance RCM, Xu P & Fincher GB (1996) Seven members of the (1-3)-β-glucanase gene family in barley are clustered on the long arm of chromosome 3 (3HL). Theor. Appl. Genet. 92: 791-796
LiWL, Faris JD, Chittoor JM, Leach JE, Liu DJ, Chen PD & Gill BS (1999) Genomic mapping of defense response genes in wheat. Theor. Appl. Genet. 98: 226-233
LinW, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S & Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Bio/Technology 13: 686-691
Linthorst HJM (1991) Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 10: 123-150
Liu D, Ragothama KG, Hasegawa PM & Bressan RA (1994) Osmotin overexpression in potato delays development of disease symptoms. Proc. Natl. Acad. Sci. USA 91: 1888-1892
Lo SC & Nicholson RL (1998a) Reduction of light-induced anthocyanin accumulation in inoculated sorghum mesocotyls. Implications for a compensatory role in the defense response. Plant Physiol. 116: 979-989
Lo SC Hipskind JD & Nicholson RL (1998b) cDNA cloning of a sorghum pathogenesis-related protein (PR-10) and differential expression of defense-related genes following inoculation with Cochliobolus heterostrophus or Colletotrichum sublineolum. Mol. Plant-Microbe Interact. 12: 479-489
Ludwig A & Boller T (1990) A method for the study of fungal growth inhibition by plant proteins. FEMS Microbiol. Lett. 69: 61-66
Lusso M & Kuc J (1995) Evidence for transcriptional regulation of β-1,3-glucanase as it relates to induced systemic resistance of tobacco to blue mold. Molec. Plant-Microbe Interact. 8(3): 473-475
Maier-Greiner UH, Obermaier-Skrobranek BMM, Estermaier LM, Kammerloher W, Freund C, Wulfing C, Burkert UI, Matern DH, Breuer M, Eulitz M, Kufrevioglu I & Hartmann GR (1991a) Isolation and properties of a nitrile hydratase from the soil fungus, Myrothecium verucaria that is highly specific for the fertilizer cyanamide and cloning of its gene. Proc. Natl. Acad. Sci. USA 88: 4260-4264
Maier-Greiner UH, Klaus CBA, Estermaier LM & Hartmann GR (1991b) Herbicide resistance in transgenic plants through degradation of the phytotoxin to urea. Angew. Chem. Chem. Int. Ed. Engl. 30: 1314-1315
Malehorn DE, Scott KJ & Shah DM (1993) Structure and expression of a barley acidic β-glucanase gene. Plant Molec. Biol. 22: 347-362
Malehorn DE, Borgmeyer JR, Smith CE & Shah DM (1994) Characterization and expression of an antifungal zeamatin-like protein (Zlp) gene from Zea mays. Plant Physiol. 106: 1471-1481
Marchant R, Davey MR, Lucas JA, Lamb CJ, Dixon RA & Power JB (1998)Expression of a chitinase transgene in rose (Rosa hybrida L.) reduces development of black spot disease (Diplocarpon rosae Wolf). Molec. Breed. 4: 187-194
Mauch F, Mauch-Mani B & Boller T (1988) Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 88: 936-942
Mauch F & Staehelin LA (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell 1: 447-457
Melchers LS, Sela-Buurlage MB, Vloemans SA et al. (1993) Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and ß-1,3-glucanase in transgenic plants. Plant Molec. Biol. 21: 583-593
Mochizuki A, Nishizawa Y, Onodera H, Tabei Y, Toki S, Habu Y, Ugaki M & Ohashi Y (1999) Transgenic rice plants expressing a trypsin inhibitor are resistant against rice stem borers, Chilo suppressalis. Entom. Exper. Appl. 93: 173-178
Moiseyev G, Beintema JJ, Fedoreyeva L et al. (1994) High sequence similarity between a ribonuclease from ginseng calluses and fungus-elicited proteins from parsley indicates that intracellular pathogenesis-related (IPR) proteins are ribonucleases. Planta 193: 470-472
Molano J, Polacheck I, Duran A & Cabib E (1979) An endochitinase from wheat germ. Activity on nascent and preformed chitin. J. Biol. Chem. 254: 4901-4907
Molodchenkova OO, Levitskii AP, Levitskii IA, Adamovskaia VG & Dymokovskaia (1998) Trypsin inhibitors of wheat seedlings infected and treated with salicylic acid. Ukr. Biokhim. Zh. 70: 30-37
Moons A, Prinsen E, Bauw G & Van Montague M (1997) Antagonistic effects of abscisic acid and jasmonates on salt stressinducible transcripts in rice roots. Plant Cell 9: 2243-2259
Munch-Garthoff S, Neuhas JM, Boller T, Kemmerling B & Kogel KH (1997) Expression of a β-1,3-glucanase and chitinase in healthy, stem-rust-affected and elicitor-treated near-isogenic wheat lines showing Sr5-or Sr24-specified race-specific rust resistance. Planta 201: 235-244
Nelson JC, Sorrels ME, Van Deynse AE, Yu YH, Atkinson M, Bernard M, Leroy P, Faris JD & Anderson JD (1995) Molecular mapping of wheat. Major genes and rearrangements in homoeologous groups 4, 4 and 7. Genetics 141: 721-731
Neuhaus JM, Ahl-Goy P, Hinz U et al. (1991a) High-level expression of a tobacco chitinase gene in Nicotiana sylvestris: Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Molec. Biol. 16: 141-151
Neuhaus JM, Sticher L, Meins F et al. (1991b) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 88: 10362-10366
Neuhas J-M (1999) Plant Chitinases (PR-3,PR-4, PR-8,PR-11). In: Datta SK & Muthukrishnan S (eds) Pathogenesis-related Proteins in Plants (pp. 77-105). CRC Press, Boca Raton, FL
Niderman T, Genetet I, Bruyere T et al. (1995) Pathogenesis-related PR-1 proteins are antifungal: Isolation and characterization of three 14 kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 108: 17-27
Nishizawa Y, Nishio Z, Nakazono K, Soma M, Nakajima E, Ugaki M & Hibi T (1999) Enhanced resistance to blast (Magnaporthe grisea) in transgenic Japonica rice by constitutive expression of rice chitinase. Theor. Appl. Genet. 99: 383-390
Pawlowski WP & Somers DA (1998) Transgenic DNA integrated into host genomes is frequently interspersed by host DNA. Proc. Natl. Acad. Sci. USA 95: 12106-12110
Posch A, Chen Z, Wheeler C et al., (1997) Characterization and identification of latex allergens by two-dimensional electrophoresis and protein microsequencing. J. Allergy Clin. Immunol. 99: 385-397
Rakwal R, Agarwal GK & Yonekura M (1999) Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by twodimensional polyacrylamide gel electrophoresis: Induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 20: 3472-3478 5mediated by Agrobacterium in
Rebmann G, Mauch F & Dudler R (1991) Sequence of a wheat cDNA encoding a pathogen-induced thaumatin-like protein. Plant Molec. Biol. 17: 283-285
Reimers PJ, Guo A & Leach JE (1992) Increased activity of a cationic peroxidase associated with an incompatible interaction between Xanthomonas oryzae pv. oryzae and rice (Oryza sativa). Plant Physiol. 99: 1044-1050
Reimmann C & Dudler R (1993) cDNA cloning and sequence analysis of a pathogen-induced thaumatin-like protein from rice (Oryza sativa). Plant Physiol. 101: 1113-1114
Repellin A, Båga M, Jauhar PP & Chibbar N (2001) Genetic enrichment of cereal crops via alien gene transfer: New challenges. Plant Cell Tiss. Org. Cult. 64: 159-183
Roberts WK & Selitrennikoff CP (1990) Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. J. Gen. Microbiol. 136: 1771-1778
Rohrmeier T & Lehle L (1993) WIP1, a wound-inducible gene from maize with homology to Bowman-Birk proteinase inhibitors. Plant Molec. Biol. 22: 783
Roulin S, Xu P, Brown AHD & Fincher GB (1997) Expression of specific (1,3)-β-glucanase genes of near-isogenic resistant and susceptible barley lines infected with the leaf scald fungus (Rhynchosporium secalis). Physiol. Molec. Plant Pathol. 50: 245-261
Sears ER (1966) Nullisomic-tetrasomic combinations in hexaploid wheat. In: Riley R & Lewis KR (eds) Chromosome Manipulation and Plant Genetics (pp. 29-45). Oliver & Boyd, Edinburgh
Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA et al. (1993) Only specific tobacco (Nicotiana tabacum) chitinases and β-glucanases exhibit antifungal activity. Plant Physiol. 101(3): 857-863
Snowden KC, Richards KD & Garner RC (1995) Aluminum-induced genes. Induction by toxic metals, low calcium, and wounding and pattern of expression in root tips. Plant Physiol. 107: 341
Somssich IE, Schmelzer E, Bollman J et al. (1986) Rapid activation by fungal elicitor of genes encoding ‘pathogenesis-related’ proteins in cultured parsley cells. Proc. Natl. Acad. Sci. USA 83: 2427-2430
Staehelin C, Schultze M, Kondorosi E et al. (1994) Structural modifications in Rhizobium meliloti Nod factors influence their stability against hydrolysis by root chitinases. Plant J. 5: 319-330
Steiglitz H (1977) Role of β-1,3-glucanase in post-meiotic microspore release. Dev. Biol. 57: 87.
Stevens C, Titarenko E, Hargreaves JA & Gurr SJ (1996) Defenserelated gene activation during an incompatible interaction between Staganospora (Septoria) nodorum and barley (Hordeum vulgare L.) coleoptile cells. Plant Molec. Biol. 31: 741-749
Svitashev S, Ananiev E, Pawlowski WP & Somers DA (2000) Association of transgene integration sites with chromosome rearrangements in hexaploid oat. Theor. Appl. Genet. (in press)
Swegle MS, Huang JK, Lee G & Muthukrishnan S (1989) Identification of an endochitinase cDNA clone from barley aleurone cells. Plant Molec. Biol. 12: 403-412
Swegle M, Kramer KJ & Muthukrishnan S (1992) Properties of barley seed chitinases and release of embryo-associated isoforms during early stages of imbibition. Plant Physiol. 99(3): 1009-1014
Tabei Y, Kitade S, Nishizawa Y, Kikuchi N, Kayano T, Hibi T & Akutsu K (1998) Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botrytis cinerea). Plant Cell Rep. 17: 159-164
Takeuchi Y, Yoshikawa M, Takeba G et al. (1990) Molecular cloning and ethylene induction of mRNA encoding a phytoalexin elicitor-releasing factor, β-1,3-endoglucanase, in soybean. Plant Physiol. 93: 673-682
Toyoda H, Matsuda Y, Yamaga T, Ikeda S, Morita M, Tamai T & Ouchi S (1991) Suppression of the powdery mildew pathogen by chitinase microinjected into barley coleoptile epidermal cells. Plant Cell Rep. 10: 217-220
Tsuchiya T, Toriyama K, Yoshikawa M et al. (1995) Tapetumspecific expression of the gene for an endo-β-1,3-glucanase causes male sterility in transgenic tobacco. Plant Cell Physiol. 36(3): 487-494
Van der Westhuizen AJ, Qian XM & Botha AM (1998) Differential induction of apoiplastic peroxidase and chitinase activities in susceptible and resistant wheat cultivars by Russian wheat aphid infestation. Plant Cell Rep. 18: 132-137
Van Loon LC & Van Kammen A (1968) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. 'samsun’ and 'samsun NN’ I. Phytochemistry 7: 1727-1735
Van Loon LC (1995) Pathogenesis-related proteins. Plant Molec. Biol. 4: 111-116
Van Loon LC (1999) Occurrence and properties of plant pathogenesis-related proteins. In: Datta SK & Muthukrishnan S (eds) Pathogenesis-related Proteins in Plants (pp. 1-19). CRC Press, Boca Raton, FL
Van Loon LC & Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. & Molec. Plant Pathol. 55: 85-97
Vasil IK (ed.) (1999) Molecular Improvement of Cereal Crops. Kluwer Academic Publishers, Dordrecht
Velazhahan R, Cole KC, Anuratha CS & Muthukrishnan S (1998) Induction of thaumatin-like proteins (TLPs) in Rhizoctonia solani-infected rice and characterization of two new cDNA clones. Physiol. Plant. 102: 21-28
Vera P & Conejero V (1988) Pathogenesis-related proteins of tomato. P-69 as an alkaline endoproteinase. Plant Physiol. 87: 58-63
Vigers AJ, Roberts WK & Selitrennikoff CP (1991) A new family of plant antifungal proteins. Molec. Plant-Microbe Interact. 4: 315-323
Vigers AJ, Wiedema S, Roberts WK et al. (1992) Thaumatin-like pathogenesis-related proteins are antifungal. Plant Sci. 83: 155-161
Warmke HE & Overman MA (1972) Cytoplasmic male sterility in sorghum. Callose behavior in fertile and sterile anthers. J. Hered. 63: 103-108
Waters EJ, Shirley NJ & Williams PJ (1996) Nuisance proteins of wine are grape pathogenesis-related proteins. J. Agric. Food Chem. 44(1): 3-5
Woloshuk, CP, Muelenhoff JS, Sela-Buurlage M, Elzen PJM & Cornelissen BJC (1991) Pathogen-induced proteins with inhibitory activity towards Phytophthora infestans. Plant Cell 3: 619-628
Worrall D, Hird DL, Hodge R et al., (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4(7): 759-771
Wu S, Kriz AL & Widholm JM (1994a) Molecular analysis of two cDNA clones encoding acidic class I chitinase in maize. Plant Physiol. 105: 1097-1105
Wu S, Kriz AL & Widholm JM (1994b) Nucleotide sequence of a maize cDNA for a class II, acidic β-1,3-glucanase. Plant Physiol. 106: 1709-1710
Xu D, Xue Q, McElroy D, Mawal Y, Hilder VA & Wu R (1996) Constitutive expression of a cowpea trypsin inhibitor gene, CpTi in transgenic rice plants confers resistance to two major rice insect pests. Molec. Breed. 2: 167-173
Xu P, Harvey AJ & Fincher GB (1994) Heterologous expression of cDNAs encoding barley (Hordeum vulgare) (1,3)-β-glucanase isozyme GV. FEBS Lett. 348: 206-210
Yoshikawa M, Tsuda M & Takeuchi Y (1993) Resistance to fungal diseases in transgenic tobacco plants expressing the phytoalexin elicitor-releasing factor, β-1,3-endoglucanase, from soybean. Naturwiss. 80(9): 417-420
Zhou J-M (1999) Signal transduction and pathogen-induced PR-protein expression. In: Datta SK & Muthukrishnan S (eds) Pathogenesis-related Proteins in Plants. (pp. 1-19). CRC Press, Boca Raton, FL
Zhu H, Krishnaveni S, Liang GH & Muthukrishnan S (1998) Biolistic transformation of sorghum using a rice chitinase gene. J. Genet. Breed. 52: 243-252
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukrishnan, S., Liang, G.H., Trick, H.N. et al. Pathogenesis-related proteins and their genes in cereals. Plant Cell, Tissue and Organ Culture 64, 93–114 (2001). https://doi.org/10.1023/A:1010763506802
Issue Date:
DOI: https://doi.org/10.1023/A:1010763506802