Abstract
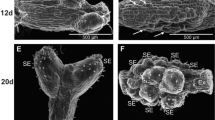
An efficient system for the in vitro plant and shootregeneration of Lilium longiflorum was developed andaccomplished using transverse thin cell layers (tTCL) of young stems.tTCLs were cut transversely along young stems from which the shoot-tipshad been removed. Sections were measured accurately using a graded gridand were cut in 4 mm × 4 mm × 1 mm cubes, eliminatingepidermal tissue, and were cultured on one-half MS medium containing 8 gl−1 agar, different sucrose concentrations (10, 20, 30 or 40g l−1), and with or without 1 mg l−1 activatedcharcoal (AC). Plants formed on the surface of tTCLs within 60 days onone-half MS medium containing 8 g l−1 agar and 20 gl−1 sucrose. Sections of 1 mm taken just below the apicalarea developed buds within 15 days, whereas the sections closer to thebase required about 45 days. Shoot regeneration was enhanced whensucrose concentration was used at 30 or 40 g l−1 after 60days of culture. No root formation occurred. Both shooting and rootingoccurred when sucrose was used at 20 g l−1. The plantletswere transferred to soil and grew well under greenhouseconditions.
Similar content being viewed by others
References
Bonnier FJM and Van Tuyl JM (1997) Long term in vitro storage of Lily: effect of temperature and concentration of nutrients and sucrose. Plant Cell Tiss Org Cult 49: 81–87
Buter B, Pescitelli SM, Berger K, Schmid JE and Stamp P (1993) Autoclaved and filter sterilized liquid media in maize anther culture: significance of activated charcoal. Plant Cell Rep 13: 79–82
Carlberg I, Glimelius K and Eriksson T (1983) Improved culture ability of potato protoplats by use of activated charcoal. Plant Cell Rep 2: 223–225
Druart P and Wulf O (1993) Activated charcoal catalyses sucrose hydrolysis during autoclaving. Plant Cell Tissue Organ Cult 32: 97–99
Dumas E and Monteuuis O (1995) In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Tissue Cell Organ Cult 40: 231–235
Ebert A and Taylor HF (1990) Assessment of changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 20: 165–172
Fridborg G, Pedersen M, Landstrom LE and Eriksson T (1978) The effects of activated charcoal on tissue culture: absorption of metabolites inhibiting morphogenesis. Physiol Plant 43: 104–106
Fuchs A (1991) The effects of activated charcoal in culture medium on the frequency and intensity of the callogenesis and organogenesis of Zea mays L and Brassica napus L. in vitro. Beitr Trop Landwirtsch Bet Med 29 H: 57–62
Gerrits MM and De Klerk GJ (1992) Dry-matter partitioning between bulb and leaves in plantlets of Lilium speciosum regenerated in vitro. Acta Bot Neerl 41: 461–468
Gupta P, Sharma AK and Charturvedi HC (1978) Multiplication of Lilium longiflorum Thumb, by asceptic culture of bulb-scales and their segments. Indian J Exp Biol 16: 940–942
Kunitake H, Nakashima T, Mori K, Tanaka M and Mii M (1995) Plant regeneration from mesophyll protoplasts of Lisianthus (Eusroma gradiflorum) by adding activated charcoal into protoplasts culture medium. Plant Cell Tissue Organ Cult 43: 59–65
Liu MSC (1993) Plant regeneration in cell suspension culture of sugarcane as affected by activated charcoal, medium composition and tissue culture. Taiwan Sugar May-June: 18–25
Mathews H, Schopke C, Carcamo R, Chavarriaga P, Fauquet C and Beachy RN (1993) Improvement of somatic embryogenesis and plant recovery in cassava. Plant Cell Rep 12: 328–333
McRae EA and McRae JF (1979) Eight years of adventure in embryo culture. Lily Yearb N Am Lily Soc 32: 74–81
Murashige T and Shoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497
Nhut DT (1998) Micropropagation of lily (Lilium longiflorum) via in vitro stem node and pseudo-bulblet culture. Plant Cell Rep 17: 913–916
Nightingale AE (1979) Bulblet formation on Lilium longiflorum Thumb. ‘Nellie white’ by foliar spray applications of PBA. HortScience 14: 67–68
Patel KR and Thorpe TA (1984) In vitro differentiation of plantlets from embryonic explants of lodge pole pine (Pinus contorta Dougl. Ex Loud.). Plant Cell Tissue Organ Cult 3: 131–142
Qu Y, Mok MC, Mok DWS and Stang JR (1988) Phenotypic and cytological variation among plants derived from anther cultures of Lilium longiflorum. In Vitro Cell. Biol 24: 471–476
Sinha RK and Mallick R (1991) Plantlets from somatic callus tissue of the wood legume Sesbania bispinosa (Jacq.) W.F.wight. Plant Cell Rep 13: 247–250
Stenberg NE, Chen CH and Ross JG (1977) Regeneration of plantlets from leaf cultures of Lilium longiflorum Thumb. Proc SD Acad Sci 56: 152–158
Stimart DP and Ascher PD (1981) Foliar emergence from bulblets of Lilium longiflorum Thunb, as related to in vitro generation temperatures. J Am Soc Hortic Sci 106: 446–450
Takayama S and Misawa M (1980) Differentiation in Lilium bulb-scale grown in vitro. Effects of activated charcoal, physiological age of bulbs and sucrose concentration on differentiation and scale leaf formation in vitro. Physiol Plant 48: 121–125
Teixeria JB, Sondahl MR and Kirby EG (1994) Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Rep 13: 247–250
Teng WL (1997) Activated charcoal affects morphogenesis and enhances sporophyte regeneration during leaf cell suspension culture of Platycerium bifurcatum. Plant Cell Rep 17: 77–83
Theander O and Nelson DA (1988) Aqueous, hightemperature transformation of carbohydrates relative to utilization of biomass. Adv Carbohydr Chem Biochem 46: 273–326
Weatherhead MA, Burdon J and Henshaw GG (1978) Some effects of activated charcoal as an additive to plant tissue culture media. Z Pflanzenphysiol 89: 141–147
Weatherhead MA, Burdon J and Henshaw GG (1979) Effects of activated charcoal as an additive plant tissue culture media. Z Pflanzenphysiol 94: 399–405
Zaghmount OMF and Torello WA (1988) Enhance regeneration in long term callus cultures of red fescus by pre-treatment with activated charcoal. HortScience 23: 615–616
Ziv M and Gadasi G (1986) Enhanced embryogenesis and plant regeneration from cucumber (Cucumis sativus L.) callus by activated charcoal in solid/liquid double-layer cultures. Plant Sci 47: 115–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nhut, D.T., Van Le, B., Fukai, S. et al. Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regulation 33, 59–65 (2001). https://doi.org/10.1023/A:1010701024963
Issue Date:
DOI: https://doi.org/10.1023/A:1010701024963