Abstract
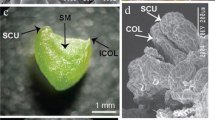
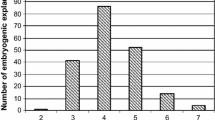
A simple, rapid and effective system to regenerate Arabidopsis plants via direct somatic embryogenesis has been established. Somatic embryogenesis was induced directly during culture of immature zygotic embryos. The frequency of somatic embryogenesis was strongly influenced by the stage of development of the explants. Explants in different developmental stages were cultured on B5 agar medium containing 5 μM 2,4-dichlorophenoxyacetic acid and the highest frequency (up to 90%) of somatic embryogenesis was observed in zygotic embryos with fully-developed cotyledons. The first somatic embryos developing directly from explant tissue were noticed after 8 days of culture. Somatic embryogenesis of a high frequency (87–96%) was observed in cultures of the all six genotypes tested (Columbia, C-24, RLD, Wassilewskaja, Landsberg erecta and Wilna). Subculture of somatic embryos onto auxin-free medium resulted in their conversion into plants with an average frequency of 79.5%. The regenerates showed normal morphological characteristics and were fertile. All 56 analysed plants displayed a diploid number of chromosomes and two out of 96 (2.1%) tested plants carried a chlorophyll or embryo-lethal mutation.
Similar content being viewed by others
References
Acedo GN (1986) Regeneration of Arabidopsis callus in vitro. Plant Cell Tiss. Org. Cult. 6: 109–114
Ahloowalia BS (1991) Somatic embryos in monocots. Their genesis and genetic stability. Rev. Cytol. Biol. Veget–Bot. 14: 223–235
Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K & Shimura Y (1992) Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep. 12: 7–11
Akashi R, Uchiyama T, Sakamato A, Kawamura O & Hoffmann F (1998) High–frequency embryogenesis from cotyledons of bird's foot trefoil (Lotus corniculatus) and its effective utilization in Agrobacterium tumefaciens–mediated transformation. J. Plant Physiol. 152: 84–91
Bhojwani SS & Razdan MK (1996) Somatic embryogenesis. In: Bhojwani SS & Razdan MK (eds) Plant Tissue Culture: Theory and Practice, a Revised Edition (pp 125–166). Elsevier, Amsterdam, New York, Oxford
Choi YE, Yang DC, Park JC, Soh WY & Choi KT (1998) Regenerative ability of somatic single and multiple embryos from cotyledons of Korean ginseng on hormone–free medium. Plant Cell Rep. 17: 544–551
Cuenca B, San–Jose MC, Martinez MT, Ballester A & Vieitez AM (1999) Somatic embryogenesis from stem and leaf explants of Ouercus robur L. Plant Cell Rep. 118: 538–543
Distabanjong K & Geneve RL (1997) Multiple shoot formation from normal and malformed somatic embryo explants of Eastern redbud (Cercis canadensis L.). Plant Cell Rep. 16: 334–338
Dodeman VL, Ducreux G & Kreis M(1997) Zygotic embryogenesis versus somatic embryogenesis. J. Exp. Bot. 48: 1493–1509
Duncan RR (1997) Tissue culture induced variation in crop improvement. In: Sparks DL (ed), Advances in Agronomy, Vol 58 (pp 201–240). Academic Press
Eady CC, Butler RC & Suo Y (1998) Somatic embryogenesis and plant regeneration from immature embryo cultures of onion (Alium cepa L.). Plant Cell Rep. 18: 111–116
Engelborghs I, Swennen R & van Campenhout S (1998) The potential of AFLP to detect genetic differences and somaclonal variants in Musa spp. InfoMusa 7: 3–6
Farooqui MA, Rao A, Jayasree V & Sadanandam A (1997) Induction of atrazine resistance and somatic embryogenesis in Solanum melongena. Theor. Appl. Genet. 95: 702–705
Feldmann KA & Marks MD (1986) Rapid and efficient regeneration of plants from explants of Arabidopsis thaliana. Plant Science 47: 63–69
Fouree JL, Barger P, Niquet L & Andre P (1997) Somatic embryogenesis and somaclonal variation in Norway spruce: morphogenetic, cytogenetic and molecular approaches. Theor. Appl. Genet. 94: 159–169
Gaj MD & Maluszynski M (1987) Genetic variation in callusderived plants of Arabidopsis thaliana (L.) Heynh. Arabidopsis Inf. Serv 23: 1–8
Gaj MD, Kucharska M, Maluszynski M & Polok K (1991) Isozyme variation in callus culture of Arabidopsis thaliana (L.) Heynh. Genetica Polonica 32: 217–225
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirement of suspension cultures of soybean root cells. Exp Cell Res. 50: 151–158
Goebel–Tourand I, Mauro MC, Sossontzov, Miginiac E & Deloire A (1993) Arrest of somatic embryo development in grapevine: histological characterization and the effect of ABA, BAP and zeatin in stimulating plant development. Plant Cell Tiss. Org. Cult. 33: 91–103
Graaff E & Hooykaas PJJ (1996) Improvements in the transformation of Arabidospsis thaliana C24 leaf–discs by Agrobacterium tumefaciens. Plant Cell Rep. 15: 572–577
Henry RJ (1998) Molecular and biochemical characterization of somaclonal variation. In: Jain SM, Brar DS & Ahloowalia BS (eds) Somaclonal Variation and Induced Mutations in Crop Improvement (pp 485–499). Kluwer Academic Publishers, Dordrecht
Henry RJ, Nato A & De Buyser J (1998) Genetic fidelity of plants regenerated from somatic embryos of cereals. In: Jain SM, Brar DS & Ahloowalia BS (eds) Somaclonal Variation and Induced Mutations in Crop Improvement (pp 65–80). Kluwer Academic Publishers, Dordrecht
Huang BC & Yeoman MM (1983) Formation of somatic embryos in tissue cultures of Arabidopsis thaliana. Arab Inf. Service 20: 73–78
Kaldenhoff R, Henningsen U & Richter G (1994) Gene activation in suspension–cultured cells of Arabidopsis thaliana during bluelight–light dependent plantlet regeneration. Planta 195: 182–187
Kintzios S, Manos C & Makri O (1999) Somatic embryogenesis from mature leaves of rose (Rosa sp.). Plant Cell Rep. 18: 467–472
Luo Y & Koop HU (1997) Somatic embryogenesis in cultured immature zygotic embryos and leaf protoplasts of Arabidopsis thaliana ecotypes. Planta 202: 387–396
Meijer EA, de Vries SC & Mordhorst AP (1999) Co–culture with Daucus carota somatic embryos reveals high 2,4–D uptake and release rates of Arabidopsis thaliana cultured cells. Plant Cell Rep. 18: 656–663
Meinke DW (1991) Perspectives on genetic analysis of plant embryogenesis. The Plant Cell 3: 857–866
Meinke DW (1995) Molecular genetics of plant embryogenesis. Annu. Rev. Physiol. Plant Mol. Biol. 46: 369–394
Mordhorst AP, Toonen MAJ & de Vries SC (1997) Plant Embryogenesis. Crit. Rev. Plant Sci. 16: 535–576
Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, VanWent J, Koornneef M & deVries SC (1998) Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutation in genes repressing meristematic cell divisions. Genetics 149: 549–563
Müller AJ (1963) Embryonentest zum Nachweis rezessiver Letafaktoren bei Arabidopsis thaliana. Biologischen Zentralblatt 82: 133–163
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 437–497
O'Neill CM & Mathias RJ (1993) Regeneration of plants from protoplasts of Arabidopsis thaliana L. cv.Columbia (C24) via direct embryogenesis. J. Exp. Bot. 44: 1579–1585
Pillon E, Terzi M, Baldan B, Mariani P & Schiavo FL (1996) A protocol for obtaining embryogenic cell lines from Arabidopsis. The Plant J. 9: 573–577
Plader W, Malepszy S, Burza W & Rusinowski Z (1998) The relationship between the regeneration system and genetic variability in the cucumber (Cucumis sativus L.) Euphytica 103: 9–15
Rival A, Bertrand L, Beule T, Combes MC, Trouslot P & Lashermes P (1998) Suitability of RAPD analysis for the detection of somaclonal variants in oil palm (Elaeis guineesis Jacq). Plant Breeding 117: 73–76
Sangwan RS, Bourgeois Y, Dubois F & Sangwan–Norreel BS (1992) In vitro regeneration of Arabidopsis thaliana from cultured zygotic embryos and analysis of regenerates. J. Plant. Physiol. 140: 588–595
Thakur RC, Goto S, Ishii K & Jain SM (1999) Monitoring genetic stability in Quercus serrata Thunb. somatic embryogenesis using RAPD markers. J. For. Res, 4: 157–160
Valvekens D, van Montagu M & van Lijsebettens M (1988) Agrobacterium tumefaciens–mediated transformation of Arabidopsis thaliana root explants by using kanamacin selection. Proc. Natl. Acad. Sci. USA 85: 5536–5540
Vendrame WA, Kochert G & Wetzstein HY (1999) AFLP analysis of variation in pecan somatic embryos. Plant Cell. Rep. 18: 853–857
Wu Y, Haberland G, Zhou C & Koop HK (1992) Somatic embryogenesis, formation of morphogenic callus and normal development in zygotic embryos of Arabidopsis thaliana in vitro. Protoplasma 169: 89–96
Yasutani I, Shoichi O, Nishida T, Sugiyama M & Kamamine A (1994) Isolation of tempereture–sensitive mutants of Arabidopsis thaliana that are defective in the redifferentation of shoots. Plant Physiol. 105: 815–822
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gaj, M.D. Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. Plant Cell, Tissue and Organ Culture 64, 39–46 (2001). https://doi.org/10.1023/A:1010679614721
Issue Date:
DOI: https://doi.org/10.1023/A:1010679614721