Abstract
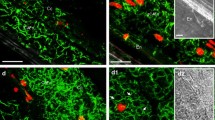
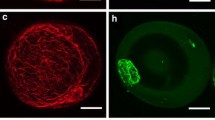
Rhodamine-phalloidin staining of winter oilseed rape suspension cells revealed that the structure of actin cytoskeleton changes with the phase of cell growth. In small, 4-day-old cells, entering the exponential phase of growth, a dense and uniformly distributed cortical microfilament networks was seen. In six-day-old vacuolated cells, which reached the stationary phase of growth, the actin cytoskeleton was composed of thicker microfilament cables in irregular arrangements. In cells acclimated in cold for 7 days a dense, uniformly distributed and cortical microfilament network was still seen. The fine microfilament network was sensitive to extracellular freezing since the structures underwent depolymerization at −3 °C (in the presence of extracellular ice), both in non-acclimated and cold-acclimated cells. The thicker transvacuolar cables in cells of the stationary growth phase resisted freezing to −7 °C. Acclimation of suspensions at 2 °C resulted in slowing down growth of cells and in the increased freezing tolerance of cells as indicated by a decrease of LT50 from −11 °C to −17.5o or to −25 °C when determined 7 or 20 days after the beginning of the cold treatment, respectively. Freezing tolerance of non-acclimated cells decreased from −11 °C to −8 °C during subculture, showing a transient increase to −17 °C on the day 6. Results indicate that the arrangement of actin microfilaments and their sensitivity to freezing-induced depolymerization depends on the phase of cell growth rather than on cell acclimation status. Possible mechanisms involved in the freezing-induced depolymerization of actin microfilaments are discussed.
Similar content being viewed by others
References
Arora R & Wisniewski ME (1995) Ultrastructural and protein changes in cell suspension cultures of peach associated with low temperature-induced cold acclimation and abscisic acid treatment. Plant Cell Tiss. Org. Cult. 40: 17-24
Bartolo ME & Carter JV (1991a) Microtubules in mesophyll cells of nonacclimated and cold-acclimated spinach. Plant Physiol. 97: 175-181
Bartolo ME & Carter JV (1991b) Effect of microtubule stabilisation on freezing tolerance of mesophyll cells of spinach. Plant Physiol. 97: 182-187
Bonner CA, Kenyon C & Jensen RA (1988) Physiological and biochemical characterization of a suspension culture system for sustained exponential growth of Nicotiana silvestris. Physiol. Plant. 74: 1-10
Borochov A, Walker MA & Pauls KP (1989) Effect of cold acclimation on the morphological and physiological properties of alfalfa (Medicago sativa) suspension cultures cells. J. Plant Physiol. 133: 671-677
Carter JV & Wick SM (1984) Irreversible microtubule depolimerisation associated with freezing injury in Allium cepa root-tip cells. Cryo Lett. 5: 175-181
Chen PM & Gusta LV (1982) Cold acclimation of wheat and smooth bromegrass cell suspensions. Can. J. Bot. 60: 1207-1211
Chu B, Kerr GP & Carter JV (1993) Stabilizing microtubules with taxacol increases microfilament stability during freezing in rye root tips. Plant Cell Environ. 16: 883-889
Danyluk J, Carpentier E & Sarhan F (1996) Identification and characterization of a low temperature regulated gene encoding an actin-binding protein from wheat. FEBS Letters 389: 324-327
Derksen J, Traas JA & Oostendorp T (1986) Distribution of actin microfilaments in differentiating cells of Equisetum hyemale root tips. Plant Science 43: 77-81
Derksen J, Wilms FHA & Pierson ES (1990) The plant cytoskeleton: its significance in plant development. Acta Bot. Neerl. 39: 1-18
Doris FP & Steer MW (1996) Effects of fixatives and permeabilisation buffers on pollen tubes: implications for localisation of actin microfilaments using phalloidin staining. Protoplasma 195: 25-36
Gungabissoon RA, Jiang CJ, Drobak BK, Maciver SK & Hussey PJ (1998) Interaction of maize actin-depolymerising factors with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 16(6): 689-696
Janmey P (1994) Phosphoinositides and calcium as regulators of cellular actin assembly and dissassembly. Annu. Rev. Physiol. 56: 169-191
Kacperska A (1989) Metabolic consequences of low temperature stress in chilling-insensitive plants. In: Li PH (ed) Low Temperature Stress Physiology in Crops (pp 27-40). CRC Press, Boca Raton, FL
Kacperska A (1999) Plant responses to low temperature: signaling pathways involved in plan acclimation. In: Margesin R & Schinner F (eds) Cold-Adapted Organisms-Ecophysiology, Enzymology, Molecular Biology (pp 79-103). Springer Verlag, Heidelberg
Kacperska A & Kulesza L (1987) Frost resistance of winter rape leaves as related to the changes in water potential and growth capability. Physiol. Plant. 71: 483-488
Kerr GP & Carter JV (1990) Relationship between freezing tolerance of root-tip cells and cold stability of microtubules in rye (Secale cereale L. cv. Puma). Plant Physiol. 93: 77-82
Knight MR, Campbell AK, Smith SM & Trewawas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524-526
Knight H, Trewawas AJ & Knight MR (1996) Cold calcium signaling in Arabidopsis involve two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489-503
Lehrer S (1981) Damage to actin filaments by glutaraldehyde: protection by tropomyosin. J. Cell Biol. 90: 459-466
Lindsmaier EM & Skoog F (1964) Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18: 100-127
López-Sáez JF & Fernández-Gómez E (1965) Partial mitotic index and phase indices. Experientia 21: 591-592
Meagher RB, McKinney EC & Kandasamy MK (1999) Isovariant dynamics expand and buffer the responses of complex systems: the diverse plant actin gene family. Plant Cell 11: 995-1005
Niki T & Sakai A (1981) Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiol. 22: 171-183
Orr W, Singh J & Brown DCW (1985) Induction of freezing tolerance in alfalfa cell suspension cultures. Plant Cell Reports 4: 15-18
Pomeroy MK & Siminovitch D (1971) Seasonal cytological changes in secondary phloem parenchyma cells in Robinia pseudoacacia in relation to cold hardiness. Can. J. Bot. 49: 787-785
Quader H, Hoffman A & Schnepf E (1989) Reorganzation of endoplasmic reticulum in epidermal cells of onion bulb scales after cold stress: Involvement of cytoskeletal elements. Planta 177: 273-280
Robertson AJ, Gusta LV, Reaney MJT & Ishikawa M (1987) Protein synthesis in bromegrass (Bromus inermis Leyss) cultured cells during the induction of frost tolerance by abscisic acid or low temperature. Plant Physiol. 84: 1331-1336
Sakai AS & Larcher W(1987) Frost Survival of Plants (pp 97-103). Springer Verlag, Berlin, Heidelberg, New York
Seagull RW, Falconer MM & Weerdenburg CA (1987) Microfilaments: dynamic, arrays in higher plant cells. J. Cell Biol. 104: 995-1004
Shiboaka H & Nagai R (1994) The plant cytoskeleton. Curr. Opin. Cell Biol. 6: 10-15
Smoleńska-Sym G & Kacperska A (1994) Phosphatidylinositol metabolism in low temperature-affected winter oilseed rape leaves. Physiol. Plant. 91: 1-8
Smoleńska-Sym G & Kacperska A (1996) Inositol 1,4,5-trisphosphate formation in leaves of winter oilseed rape plants in response to freezing, tissue water potential and abscisic acid. Physiol. Plant. 96: 692-698
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastr. Res. 26: 31-43
Staiger CJ, Gibbon BC, Kovar DR & Zonia LE (1997) Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 2: 275-281
Staiger CJ & Schliwa M (1987) Actin localization and function in higher plants. Protoplasma 141: 1-12
Tanino KK, Chen THH, Fuchigami LH & Weiser CJ (1991) Abscisic acid-induced cellular alterations during the induction of freezing tolerance in bromegrass cells. J. Plant Physiol. 137: 619-624
Tiwari SC, Wick SM, Williamson RE & Gunning BES (1984) Cytoskeleton and integration of cellular function in cells of higher plants. J. Cell Biol. 99: 63-69
Towill LE & Mazur P (1975) Studies on the reduction of 2,3,5-triphenyltetrazolium chloride as a vaiability assay for plant tissue culture. Can. J. Bot. 53: 1097-1102
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J & Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105: 387-395
Wallin M & Stromberg E (1995) Cold-stable and cold-adapted microtubules. Int. Rev. Cytol. 157: 1-31
Wallner SJ, Wu MT & Anderson-Krengel S J (1986) Changes in extracellular polysaccharides during cold acclimation of cultures pear cells. J. Amer. Hort. Sci. 111: 769-773
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Egierszdorff, S., Kacperska, A. Low temperature effects on growth and actin cytoskeleton organisation in suspension cells of winter oilseed rape. Plant Cell, Tissue and Organ Culture 65, 149–158 (2001). https://doi.org/10.1023/A:1010645607789
Issue Date:
DOI: https://doi.org/10.1023/A:1010645607789