Abstract
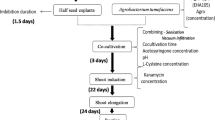
Stable transformation and regeneration was developed for a grain legume, azuki bean (Vigna angularis Willd. Ohwi & Ohashi). Two constructs containing the neomycin phosphotransferase II gene (nptII) and either the β-glucuronidase (GUS) gene or the modified green fluorescent protein [sGFP(S65T)] gene were introduced independently via Agrobacterium tumefaciens-mediated transformation. After 2 days of co-cultivation on MS medium supplemented with 100 μM acetosyringone and 10 mg l−1 6-benzyladenine, seedling epicotyl explants were placed on regeneration medium containing 100 mg l−1 kanamycin. Adventitious shoots developing from explant calli were excised onto rooting medium containing 100 mg l−1 kanamycin. Rooted shoots were excised and repeatedly selected on the same medium containing kanamycin. Surviving plants were transferred to soil and grown in a green house to produce viable seeds. This process took 5 to 7 months after co-cultivation. Molecular analysis confirmed the stable integration and expression of foreign genes.
Similar content being viewed by others
References
An G (1985) High efficiency of transformation of cultured tobacco cells. Plant Physiol. 79: 568–570
Bean SJ, Gooding PS & Mullineaux PM (1997) A simple system for pea transformation. Plant Cell Rep. 16: 513–519
Binns AN & Thomashow MF (1988) Cell biology of Agrobacterium infection and transformation of plants. Ann. Rev. Microbiol. 42: 575–606
Chang SJC, Doubler TW, Kilo V, Suttner R, Klein J, Schmidt ME, Gibson PT & Lightfoot DA (1996) Two additional loci underlying durable field resistance to soybean sudden death syndrome (SDS). Crop Sci. 36: 1684–1688
Chiu W–L, Niwa Y, Zeng W, Hirano T, Kobayashi H & Sheen J (1996) Engineered GFP as a vital reporter in plants. Current Biology 6: 325–330
Davies DR, Hamilton J & Mullineaux PM(1993) Transformation of peas. Plant Cell Rep. 12: 180–183
Di R, Purcell V, Collins GB & Ghabrial SA (1996) Production of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene. Plant Cell Rep. 15: 746–750
Dillen W, Clercq J, Goossens A, van Montagu M & Angenon G (1997) Agrobacterium–mediated transformation of Phaseolus acutifolius A. Gray. Theor. Appl. Genet. 94: 151–158
Draper J & Scott R (1988) The isolation of plant nucleic acids. In: Draper J, Scott R, Armitage P & Walden R (eds) Plant Genetic Transformation and Gene Expression (pp 212–214). Blackwell Scientific Publications, London
Hiei Y, Ohta S, Komari T & Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L) mediated by Agrobacterium and sequence analysis of the boundaries of the T–DNA. Plant J. 6: 271–282
Hood EE, Helmer GL, Fraley RT & Chilton MD (1984) Restriction endonuclease map of pTiBo542, a potential Ti plasmid vector for genetic engineering of plants. Bio/Technol. 2: 702–709
Hood EE, Gelvin SB, Melchers LS & Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgen. Res. 2: 208–218
Iida A, Yamashita Y, Yamada Y & Morikawa H (1991) Efficiency of particle–bombardment–mediated transformation is influenced by cell stage in synchronized cultured cells of tobacco. Plant Physiol. 97: 1585–1587
Ishimoto M, Sato T, Chrispeels MJ & Kitamura K (1996) Bruchid resistance of transgenic azuki bean expressing seed α–amylase inhibitor of common bean. Entomol. Exp. Appl. 79: 309–315
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5: 387–405
Jin S, Komari T, Gordon MP & Nester EW (1987) Genes responsible for the supervirlence phenotype of Agrobacterium tumefaciens A281. J. Bacteriol. 169: 4417–4425
Karthikeyan AS, Sarma KS & Veluthambi K (1996) Agrobacterium tumefaciens–mediated transformation of Vigna mungo L. Hepper. Plant Cell Rep. 15: 328–331
Kudirka DT, Colburn SM, Hinchee MA & Wright MS (1986) Interactions of Agrobacterium tumefaciens with soybean (Glycine max L. Merr.) leaf explants in tissue cultures. Can. J. Genet. Cytol. 28: 808–817
Lazo GR, Stein PA & Ludwig RA (1991) A DNA transformationcompetent Arabidopsis genomic library in Agrobacterium. Bio/Technol. 9: 963–967
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473–497
Ohta S, Mita S, Hattori T & Nakamura K (1990) Contruction and expression in tobacco of a β–glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol. 31: 805–813
Pigeaire A, Abernethy D, Smith PM, Simpson K, Fletcher N, Lu CY, Atkins CA & Cornish E (1997) Transformation of a grain legume (Lupinus angustifolius L.) via Agrobacterium tumefaciens–mediated gene transfer to shoot apices. Mol. Breed. 3: 341–349
Polhill RM & Raven PH (1981) Advances in Legume Systematics. Royal Botanic Gardens, Kew
Prabhu RR, Njiti VN, Bell–Johnson B, Johnson JE, Schmidt ME, Klein JH & Lightfoot DA (1999) Selecting soybean cultivars for dual resistance to soybean cyst nematode and sudden death syndrome using two DNA markers. Crop Sci. 39: 982–987
Sato T, Asaka D, Harada T & Matsukawa I (1990) Plant regeneration from azuki bean epicotyl and callus derived from epicotyl (in Japanese with English abstract). Bulletin Hokkaido Prefect. Agr. Exp. Stations 61: 51–60
Schroeder HE, Schotz AH, Wardley–Richardson T, Spencer D & Higgins TJV (1993) Transformation and regeneration of two cultivars of pea (Pisum sativum L.). Plant Physiol. 101: 751–757
Stachel SE, Messens E, Montagu MV & Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T–DNA transfer in Agrobacterium tumefaciens. Nature 318: 624–629
Wullems GJ, Molendijik L, Ooms G & Schilperoort RA (1981) Differential expression of crown gall tumor markers in transformants obtained after in vitro Agrobacterium tumefaciensinduced transformation of cell–wall–regenerating protoplasts derived from Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 78: 4344–4348
Zambryski PC (1992) Chronicles from the Agrobacterium–plant cell DNA transfer story. Ann. Rev. Plant Physiol. 43: 465–490
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamada, T., Teraishi, M., Hattori, K. et al. Transformation of azuki bean by Agrobacterium tumefaciens. Plant Cell, Tissue and Organ Culture 64, 47–54 (2001). https://doi.org/10.1023/A:1010635832468
Issue Date:
DOI: https://doi.org/10.1023/A:1010635832468