Abstract
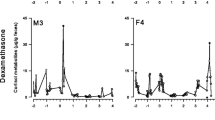
The aim of this comparative study was to gain more information about the metabolism and excretion of glucocorticoids in cats and dogs in order to establish non-invasive methods for evaluating stressful conditions. Therefore, in a first experiment, [14C]cortisol was administered intravenously to 8 animals (two of each sex and species). Over a period of 6 days, faeces and urine were collected immediately after spontaneous defecation and urination. Marked species differences were found, as cats mainly excreted cortisol in the faeces (82%±4% of the total recovered radioactivity), whereas in dogs only a small portion was found there (23%±4%). The highest urinary radioactivity was observed after 9±3 h in cats and 3±1 h in dogs. Peak concentrations in the faeces occurred after 22±6 h in cats and after 24±4 h in dogs. Most of the radioactivity was not extractable with diethyl ether, indicating that the metabolites excreted in urine and faeces were mainly of the conjugated or polar unconjugated types. This was confirmed by RP-HPLC, which also revealed marked differences between cats and dogs concerning the metabolites formed. In addition, the immunoreactivity of the metabolites was tested in cortisol, corticosterone and 11-oxoaetiocholanolone EIAs. The latter, measuring 11,17-dioxoandrostanes (11,17-DOA) detected the highest quantities of immunoreactive metabolites in cats, but not in dogs. In a second experiment, the adrenal cortex of both species was stimulated by ACTH and, three weeks later, suppressed by dexamethasone. In this study, only faeces were collected over a period of 7 days. In both species, inter-animal variability in the basal and maximal/minimal faecal cortisol metabolite concentrations and the time course was observed. The 11-oxoaetiocholanolone EIA in cats and the cortisol EIA in dogs proved best suited for monitoring changes in adrenocortical activity. ACTH injections resulted in an increase above baseline values of 355% (median) in 11,17-DOA concentrations in cats and of 702% in the concentrations of cortisol equivalents in dogs by about 25 h and 22 h (median) after injection, respectively. Minimal concentrations after dexamethasone administration were about 17% in cats and 31% in dogs (in relation to baseline values) and were reached in 66 h and 72 h, respectively. It was concluded that measuring cortisol metabolites in faeces should be a useful non-invasive tool for monitoring stress in carnivores.
Similar content being viewed by others
REFERENCES
Beerda, B., Schilder, M., Janssen, N. and Mol, J., 1996. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Hormones and Behaviour, 30, 272–279
Beerda, B., Schilder, M.B.H., Bernadina, W., Van Hoof, J.A.R.A.M., De Vries, H.W. and Mol, J.A., 1999. Chronic stress in dogs subjected to social and spatial restriction. 2. Hormonal and immunological responses. Physiology and Behavior, 66, 243–254
Bokkenheuser, V.D., Winter, J., Morris, G.N. and Locasio, S., 1986. Steroid desmolase synthesis by Eubacterium desmolans and Clostridium cadavaris. Applied Microbiology, 52, 1153–1156
Brownie, A.C., 1992. The metabolism of adrenal cortical steroids. In: V.H.T. James (ed.), The Adrenal Gland, 2nd edn, (Raven Press, New York), 209–224
Carlstead, K., Brown, J.L., Monfort, S.L., Killens, R. and Wildt, D.E., 1992. Urinary monitoring of adrenal responses to psychological stressors in domestic and nondomestic felids. Zoo Biology, 11, 165–176
Carlstead, K., Brown, J.L. and Seidensticker, J., 1993. Behavioral and adrenocortical responses to environmental changes in leopard cats (Felis bengalensis). Zoo Biology, 12, 321–331
Church, D.B., Nicholson, A.I., Ilkiw, J.E. and Emslie, D.R., 1994. Effect of non-adrenal illness, anaesthesia and surgery on plasma cortisol concentrations in dogs. Research in Veterinary Science, 56, 129–131
Clark, J.D., Rager, D.R. and Calpin, J.P., 1997a. Animal well-being: I. General considerations. Laboratory Animal Science, 47, 564–570
Clark, J.D., Rager, D.R. and Calpin, J.P., 1997b. Animal well-being: IV. Specific assessment criteria. Laboratory Animal Science, 47, 586–597
Clark, J.D., Rager, D.R., Crowell-Davis, S. and Evans, D.L., 1997c. Housing and exercise of dogs: effects on behavior, immune function, and cortisol concentration. Laboratory Animal Science, 47, 500–510
Fox, S.M., Mellor, D.J., Firth, E.C., Hodge, H. and Lawoko, C.R.O., 1994. Changes in plasma cortisol concentrations before, during and after analgesia, anaesthesia and anaesthesia plus ovariohysterectomy in bitches. Research in Veterinary Science, 57, 110–118
Gold, N.I., 1960. Partial characterization of the metabolites of cortisol-4-C14 in the dog. Journal of Biological Chemistry, 236, 1930–1933
Goossens, M.M.C., Meyer, H.P., Voorhout, G. and Sprang, E.P.M., 1995. Urinary excretion of glucocorticoids in the diagnosis of hyperadrenocorticism in cats. Domestic Animal Endocrinology, 12, 355–362
Goymann, W., Möstl, E., Van't Hof, T., East, M.L. and Hofer, H., 1999. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas (Crocuta crocuta). General and Comparative Endocrinology, 114, 340–348
Graham, L.H. and Brown, J.L., 1996. Cortisol metabolism in the domestic cat and implications for noninvasive monitoring of adrenocortical function in endangered felids. Zoo Biology, 15, 71–82
Hennessy, M.B., Davis, H.N., Williams, M.T., Mellott, C. and Douglas, C.E., 1997. Plasma cortisol levels of dogs at a county animal shelter. Physiology and Behavior, 62, 485–490
Hennessy, M.B., Williams, M.T., Miller, D.M., Douglas, C.W. and Voith, V.L., 1998. Influence of male and female petters on plasmid cortisol and behaviour: can human interaction reduce the stress of dogs in a public animal shelter. Applied Animal Behaviour Science, 61, 63–77
Jurke, M.H., Czekala, N.M., Lindburg, D.G. and Millard, S.E., 1997. Fecal corticoid metabolite measurement in cheetah (Acinonyx jubatus). Zoo Biology, 16, 133–147
Kaplan, A.J., Peterson, M.E. and Kemppainen, R.J., 1995. Effects of disease on the result of diagnostic tests for use in detecting hyperadrenocorticism in dogs. Journal of the American Veterinary Medical Association, 207, 445–451
Macdonald, I.A., Bokkenheuser, V.D., Winter, J., McLernon, A.M. and Mosbach, E.H., 1983. Degradation of steroids in the human gut. Journal of Lipid Research, 24, 675–700
McDonald, L.E., 1980. Veterinary Endocrinology and Reproduction, 3rd edn, (Lea and Febinger, Philadelphia)
Moe, R.O. and Bakken, M., 1997. Effects of handling and physical restraint on rectal temperature, cortisol, glucose and leucocyte counts in the silver fox (Vulpes vulpes). Acta Veterinaria Scandinavica, 38, 29–39
Monfort, S.L., Mashburn, K.L., Brewer, B.A. and Creel, S.R., 1998. Evaluating adrenal activity in African wild dogs (Lycaon pictus) by fecal corticosteroid analysis. Journal of Zoo and Wildlife Medicine, 29, 129–133
Möstl, E., Messmann, S., Bagu, E., Robia, C. and Palme, R., 1999. Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. Journal of Veterinary Medicine A, 46, 621–632
Ogburn, P., Crouse, S., Martin, F. and Houpt, K., 1998. Comparison of behavioral and physiology responses of dogs wearing two different types of collars. Applied Animal Behaviour Science, 61, 133–142
Palme, R. and Möstl, E., 1994. Biotin-streptavidin enzyme immunoassay for the determination of oestrogens and androgens in boar faeces. In: S. Görög (ed.) Advances of Steroid Analysis '93, (Akadémiai Kiadó, Budapest), 111–117
Palme, R. and Möstl, E., 1997. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. International Journal of Mammalian Biology, 62(supplement 2), 192–197
Palme, R., Fischer, P., Schilddorfer, H. and Ismail, M.N., 1996. Excretion of infused 14C-steroid hormones via faeces and urine in domestic livestock. Animal Reproduction Science, 43, 43–63
Palme, R., Möstl, E., Brem, G., Schellander, K. and Bamberg, E., 1997. Faecal metabolites of infused 14C-progesterone in domestic livestock. Reproduction in Domestic Animals, 32, 199–206
Palme, R., Robia, C., Messmann, S., Hofer, J. and Möstl, E., 1999. Measurement of faecal cortisol metabolites in ruminants: a non-invasive parameter of adrenal function. Wiener Tierärztliche Monatsschrift, 86, 237–241
Palme, R., Robia, C., Baumgartner, W. and Möstl, E., 2000. Transport stress in cattle as reflected by an increase in faecal cortisol metabolites. The Veterinary Record, 146, 108–109
Rijnberk, A. and Mol, J.A., 1989. Adrenocortical function. In: J.J. Kaneko (ed.) Clinical Biochemistry of Domestic Animals, 4th edn, (Academic Press, San Diego), 610–629
Smith, J.D., Allen, S.W. and Quandt, J.E., 1999. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables. American Journal of Veterinary Research, 60, 432–436
Taylor, W., 1971. The excretion of steroid hormone metabolites in bile and feces. Vitamins and Hormones, 29, 201–285
Terio, K.A., Citino, S.B. and Brown, J.L., 1999. Fecal cortisol metabolite analysis for noninvasive monitoring of adrenocortical function in the cheetah (Acinonyx jubatus). Journal of Zoo and Wildlife Medicine, 30, 484–491
Teskey-Gerstl, A., Bamberg, E., Steineck, T. and Palme, R., 2000. Excretion of corticosteroids in urine and faeces of hares (Lepus europaeus). Journal of Comparative Physiology B, 170, 163–168
van Vonderen, I.K., Kooistra, H.S. and Rijnberk, A., 1998. Influence of veterinary care on the urinary corticoid:creatinine ratio in dogs. Journal of Veterinary Internal Medicine, 12, 431–435
Vincent, I.C. and Michell, A.R., 1992. Comparison of cortisol concentrations in saliva and plasma of dogs. Research in Veterinary Science, 53, 342–345
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schatz, S., Palme, R. Measurement of Faecal Cortisol Metabolites in Cats and Dogs: A Non-invasive Method for Evaluating Adrenocortical Function. Vet Res Commun 25, 271–287 (2001). https://doi.org/10.1023/A:1010626608498
Issue Date:
DOI: https://doi.org/10.1023/A:1010626608498