Abstract
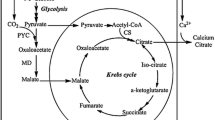
The comparative studies performed in this work showed that overproduction of α-ketoglutaric acid (KGA) and citric acid (CA) from ethanol by the mutantYarrowia lipolyticastrain 1 requires both a deficiency of thiamine and a relatively high concentration of ammonium ions in the medium, whereas CA overproduction requires an almost zero concentration of ammonium ions. The threshold value of the dissolved oxygen concentration in the medium, pO2, for CA overproduction is considerably higher than for KGA overproduction. The respiration rate of CA-overproducing cells was 2–3.5 times higher than that of KGA-overproducing cells. The main terminal electron carrier functioning in the KGA-overproducing cells was cytochrome oxidase. In the CA-overproducing cells, the main terminal oxidase was presumably o-type cytochrome.
Similar content being viewed by others
References
Chernyavskaya, O.G., Shishkanova, N.V., and Finogenova, T.V., Biosynthesis of α-Ketoglutaric Acid from Ethanol by Yeasts, Prikl. Biokhim. Mikrobiol., 1997, vol. 33, no. 3, pp. 296–300.
Il'chenko, A.P., Shishkanova, N.V., Chernyavskaya, O.G., and Finogenova, T.V., Role of Oxygen Concentration in the Regulation of the Central Metabolism Enzymes and of the Biosynthesis of Citric Acid by the Ethanol-grown Yarrowia lipolytica, Mikrobiologiya, 1998, vol. 67, no. 3, pp. 293–297.
Shishkanova, N.V., Isolation of the Candida lipolytica 704 Mutants, Prikl. Biokhim. Mikrobiol., 1979, vol. 15, no. 4, pp. 555–559.
Von Korff, R.W., Purity and Stability of Pyruvate and α-Ketoglutarate, Methods Enzymol., Colowick, S.P. and Kaplan, N.O., Eds., New York: Academic, 1969, vol. 13, p. 519.
Stern, J.R., Assay of Tricarboxylic Acids, Methods Enzymol., Colowick, S.P. and Kaplan, N.O., Eds., New York: Academic, 1957, vol. 3, pp. 425–431.
Zhabolovskaya, N.A., Ageev, L.M., and Petrova, L.F., Comparative Evaluation of Methods for the Quantitative Assay of Citric Acid, Khlebopekarn. Konditersk. Promst., 1968, no. 5, pp. 22–24.
Yonetani, T., Cytochrome c Oxidase of Beef Heart, Methods Enzymol., Colowick, S.P. and Kaplan, N.O., Eds., New York: Academic, 1967, vol. 10, pp. 332–335.
Yonetani, T., Cytochrome c Peroxidase (Baker's Yeast), Methods Enzymol., Colowick, S.P. and Kaplan, N.O., Eds., New York: Academic, 1967, vol. 10, pp. 336–339.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., Protein Measurement with Folin Phenol Reagent, J. Biol. Chem., 1951, vol. 193, no. 1, pp. 265–275.
Finogenova, T.V., Shishkanova, N.V., Fausek, E.A., and Eremina, S.S., Biosynthesis of Isocitric Acid from Ethanol by Yeasts, Appl. Microbiol. Biotechnol., 1991, vol. 36, pp. 231–235.
Yonetani, T. and Ray, G.S., Studies on Cytochrome c Peroxidase: Purification and Some Properties, J. Biol. Chem., 1965, vol. 240, no. 11, pp. 4503–4508.
Oshino, R., Oshino, N., and Chance, B., Studies on Yeast Hemoglobin: The Properties of Yeast Hemoglobin and Its Physiological Function in the Cell, Eur. J. Biochem., 1973, vol. 35, pp. 23–33.
Oshino, R., Asakura, T., Takio, K., Oshino, N., and Chance, B., Purification and Molecular Properties of Yeast Hemoglobin, Eur. J. Biochem., 1973, vol. 39, pp. 581–590.
Schultz, B.E. and Chan, S.I., Thermodynamics of Electron Transfer in Escherichia coli Cytochrome bo 3, Proc. Natl. Acad. Sci. USA, 1998, vol. 95, pp. 11643–11648.
Calhoun, M.W., Thomas, J.W., and Gennis, R.B., The Cytochrome Oxidase Superfamily of Redox-driven Proton Pumps, Trends Biochem. Sci., 1994, vol. 19, pp. 325–330.
Gohlke, U., Warne, A., and Saraste, M., Projection Structure of the Cytochrome bo Ubiquinol Oxidase from E. coli at 6 Å Resolution, EMBO J., 1997, vol. 16, pp. 1181–1188.
Siegel, L.M., Murphy, M.J., and Kamin, H., Siroheme: Methods of Isolation and Characterization, Methods Enzymol., Fleischer, S. and Packer, L., Eds., Academic, 1978, vol. LII, pp. 436–447.
Baroncelli, V., Bocealou, Y., Giannini, Y., and Renzi, P., An Inducible n-Alkane Hydroxylase System Containing Cytochrome o from Candida lipolytica, Mol. Cell Biochem., 1979, vol. 28, no. 1, pp. 3–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Il'chenko, A.P., Chernyavskaya, O.G., Shishkanova, N.V. et al. Metabolic Characteristics of the Mutant Yarrowia lipolytica Strain 1 Producing α-Ketoglutaric and Citric Acids from Ethanol and the Effect of [NH+4] and [O2] on Yeast Respiration and Acidogenesis. Microbiology 70, 151–157 (2001). https://doi.org/10.1023/A:1010421328285
Issue Date:
DOI: https://doi.org/10.1023/A:1010421328285