Abstract
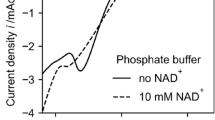
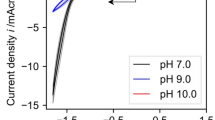
The optimal concentrations of diaphorase, methyl viologen (MV2+) and NAD+ in the mediated electrocatalytic reduction of NAD+ were decided by applying cyclic voltammetry. The steady-state catalytic current was achieved under the conditions of 1.5 U diaphorase ml−1, 0.2 mM MV2+, and 4.8 mM NAD+ at the scan rate of 2 mV s−1, suggesting that the rate of the electrocatalytic reaction is the highest under the former conditions. However, NAD+ was effective at 0.3 mM as it was at 4.8 mM when the electrocatalysis is coupled with an enzymatic synthesis requiring NADH. In effect, the electrochemical procedure under the conditions of 1.5 U diaphorase ml−1, 0.2 mM MV2+, and 0.3 mM NAD+ worked satisfactorily to drive an enzymatic reduction of pyruvate to d-lactate in the presence of d-lactate dehydrogenase.
Similar content being viewed by others
References
Aizawa M, Coughlin RW, Charles M (1976) Electrolytic regeneration of the reduced from the oxidized form of immobilized NAD. Biotechnol. Bioeng. 18: 209-215.
Baik SH, Kang C, Jeon IC, Yun SE (1999) Direct electrochemical regeneration of NADH from NAD+ using cholesterol-modified gold amalgam electrode. Biotechnol. Tech. 13: 1-5.
Bard AJ, Faulkner LR (1980) Electrochemical Methods. New York: Wiley, pp. 455-461.
Coury Jr LA, Oliver BN, Egekeze JO, Sosnoff CS, Brumfield JC, Buck RP, Murray RW (1990) Mediated anaerobic voltammetry of sulfite oxidase. Anal. Chem. 62: 452-458.
Coury Jr LA, Murray RW, Johnson JL, Rajagopalan KV (1991) Electrochemical study of kinetics of electron transfer between synthetic electron acceptors and reduced molybdoheme protein sulfite oxidase. J. Phys. Chem. 95: 6034-6040.
DiCosimo R, Wong C-H, Daniel L, Whitesides GM (1981) Enzyme-catalyzed organic synthesis: electrochemical regeneration of NAD(P)H using methyl viologen and flavoenzymes. J. Org. Chem. 46: 4622-4623.
Fisher RJ, Fenton JM, Iranmahboob J (2000) Electroenzymatic synthesis of lactate using electron transfer chain biomimetic membranes. J. Membr. Sci. 177: 17-24.
Kim SH, Yun SE, Kang C (1999) Electrochemical evaluation of the reaction rate between methyl viologen mediator and diaphorase enzyme for the electrocatalytic reduction of NAD+ and digital simulation for its voltammetric responses. J. Electroanal. Chem. 465: 153-159.
Leonida MD, Sobolov SB, Fry AJ (1998) FAD-mediated enzymatic conversion of NAD+ to NADH: application to chiral synthesis of L-lactate. Bioorg. Med. Chem. Lett. 8: 2819-2824
Maeda H, Kajiwara S (1985) Malic acid production by an electrochemical reduction system combined with the use of diaphorase and methyl viologen. Biotechnol. Bioeng. 27: 596-602.
Takagi K, Kano K, Ikeda T (1998) Mediated bioelectrocatalysis based on NAD-related enzymes with reversible characteristics. J. Electroanal. Chem. 445: 211-219.
Yun SE, Taya M, Tone S (1994) Direct reduction of NAD+ by electrochemical procedure and application of the regenerated NADH to enzyme reaction. Biotechnol. Lett. 16: 1053-1058.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kang, YW., Kang, C., Hong, JS. et al. Optimization of the mediated electrocatalytic reduction of NAD+ by cyclic voltammetry and construction of electrochemically driven enzyme bioreactor. Biotechnology Letters 23, 599–604 (2001). https://doi.org/10.1023/A:1010316708080
Issue Date:
DOI: https://doi.org/10.1023/A:1010316708080