Abstract
This study reports effects on soil solution chemistry and plant uptake of 55 elements (Ag, Al, As, B, Ba, Be, Bi, Br, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Gd, Ge, Hf, Hg, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, P, Pb, Pr, Rb, S, Sb, Sc, Se, Si, Sm, Sr, Tb, Th, Tl, U, V, W, Y, Yb, Zn, Zr) by raising the pH using addition of fine-grained precipitated calcium carbonate at 20 rates (yielding a soil solution pH range of 5.2 – 7.8) to A horizon samples of an acid Cambisol, cultivating a common grass (Agrostis capillaris L.) and determining the soil solution, root and shoot concentrations of these elements at the end of the experiment. For many of these elements, there is little or no previous information about concentrations in soil solutions, or in plant biomass, as related to soil pH/acidity or addition of calcium carbonate. Soil solutions were obtained by high speed centrifugation and ultrafiltration (0.2 μm) of samples at 60% water-holding capacity. Concentrations of elements were determined by ICP-ES or (in most elements) ICP-MS, using isotopes specified. Soil solution pH, HCO3 and organic C were also determined.
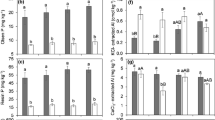
Concentrations of elements in the biomass of A. capillaris were usually inversely related to soil solution pH. The most apparent (p<0.001) inverse, though often curvilinear, relationships between pH and concentrations in shoot biomass were measured for Ag, As, B, Ba, Eu, Ge, Li, Mn, Ni, P and Sr. Positive relationships (p<0.05) were only measured in Ca, Hg, Mg, Mo and S. For concentrations in root biomass, relationships were mostly, but not always, of the same sign and of a similar strength. Though soil solution pH and concentrations of elements were usually quite closely correlated, pH and/or HCO− 3 concentration more often accounted for a higher share of the variability in biomass concentration of elements than did soil solution concentration of the same element.
Similar content being viewed by others
References
Andersson A and Siman G 1991 Levels of cadmium and some other trace elements in soils and crops as influenced by lime and fertilizer level. Acta Agric. Scand. 41, 3-12.
Berggren D 1989 Speciation of aluminium, cadmium, copper and lead in humic soil solutions. A comparison of the ion exchange column procedure and equilibrium dialysis. International J. Environ. Anal. Chem. 35, 1–15.
Brallier S, Harrison R B, Henry C L and Dongsen X 1996 Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Wat. Air Soil Pollut. 86, 195–206.
Branca M, Micera G, Dessi A and Sanna D 1990 Complexation of oxovanadium-IV by humic and tannic acids. J. Inorg. Chem. 39, 109–116.
Curtin D and Smillie G W 1983 Soil solution composition as affected by liming and incubation. Soil Sci. Soc. Am. J. 47, 701–707.
Curtin D and Smillie G W 1986 Effects of liming on soil chemical characteristics and grass growth in laboratory and long-term field-amended soils. I. Soil chemistry. Plant Soil 95, 15–22.
Dahlgren R A, Percival H J and Parfitt R L 1997 Carbon dioxide degassing effects on soil solutions collected by centrifugation. Soil Sci. 162, 648–655.
Diatloff E, Asher C J and Smith F W 1996 Concentrations of rare earth elements in some Australian soils. Austr. J. Soil Res. 34, 735–747.
Dijkshoorn W, Lampe J E M and Van Broekhoven L W 1983 Effect of soil pH and chemical form of nitrogen fertilizer on heavy metal contents in rye grass. Fert. Res. 4, 63–74.
Drobner U and Tyler G 1998 Conditions controlling relative uptake of potassium and rubidium by plants from soils. Plant Soil 201, 285–293.
Duff MC and Amrhein C 1996 Uranium( VI) adsorption on goethite and soil in carbonate solutions. Soil Sci. Soc. Amer. J. 60, 1393–1400.
Duff M C, Hunter D B, Bertsch P M and Amrhein C 1999 Factors influencing uranium reduction and solubility in evaporation pond sediments. Biogeochem. (Dordrecht) 45, 95–114.
Ebbs S D, Brady D J and Kochian L V 1998 Role of uranium speciation in the uptake and translocation of uranium by plants.J. Exp. Bot. 49 1183–1190.
Edmeades D C, Smart C E, Wheeler DM and Rys G 1983 Effects of lime on the chemical composition of ryegrass, Lolium perenne, and white clover, Trifolium repens, grown on a yellow-brown loam. N. Z. J. Agric. Res. 26, 473–482.
Elless M P and Lee S Y 1998 Uranium solubility of carbonate-rich uranium-contaminated soils. Wat. Air Soil Pollut. 107, 147–162.
Ervio R and Sippola J 1993 Micronutrient concentration of Italian ryegrass (Lolium multiflorum L.) grown on different soils in a pot experiment. Agric. Sci. Finl. 2, 141–148.
FAO-Unesco 1997 Soil map of the world. Revised edition. International Soil Reference and Information Centre; Technical Paper 20. Wageningen. 140 pp.
Fernandes M L, Abreu M M, Calouro F and Vaz M C 1999 Effect of liming and cadmium application in an acid soil on cadmium availability to sudangrass. Comm. Soil Sci. Plant Anal. 30, 1051–1062.
Gambus F 1987 The uptake of copper by various species of plants as depending on soil pH and other soil properties. Acta Agrar. Silv., Ser. Agrar. 26, 93–108.
Giesler R, Lundström U S and Grip H 1996 Comparison of soil solution chemistry assessment using zero-tension lysimeters or centrifugation.Eur. J. Soil Sci. 47, 395–405.
Glabiszewski J, Hrynczuk B and Kiepul J 1984 Content of strontium90 and cesiuml37 in some crops as dependent on soil type and time of harvest. Pamiet Pulawski (84), 205–218 (abstract).
Gorlach E, Gorlach K and Stepiens S 1980 The effect of liming and magnesium on the growth of Italian rye grass and the content of micro elements in plants and their soluble forms in the soil. Acta Agrar. Silv., Ser. Agrar. 19, 31–48.
Gorlach E, Gambus F and Michniak A 1990 The effect of pH on the uptake of heavy metals by Italian ryegrass, Lolium multiflorum, in the conditions of their differentiated contents in soil. Pol. J. Soil Sci. 23, 17–24.
Greenwood N N and Earnshaw A 1984 Chemistry of the elements. Pergamon Press, Oxford/Exeter.
Haddad K S and Kaldor C J 1984 Boron supply power, boron adsorption capacity and productivity of some acidic soils from the central table lands of New South Wales, Australia. Aust. J. Exp. Agric. Anim. Husb. 24, 120–125.
Han D H and Lee J H 1996 Effects of liming on uptake of lead and cadmium by Raphanus sativa. Archiv. Environ. Contam. Toxicol. 31, 488–493.
Hansson L, Johansson K, Olin Å and Siman G 1994 Method for the determination of selenium in low-selenium grain and the effect of liming on the uptake of selenium by barley and oats. Acta Agric. Scand., Sect.B 44, 193–200.
Helyar K R and Anderson A J 1974 Effects of calcium carbonate on the availability of nutrients in an acid soil. Soil Sci. Soc. Am. Proc. 38, 341–346.
Hooda P S and Alloway B J 1996 The effect of liming on heavy metal concentrations in wheat, carrots and spinach grown on previously sludge-applied soils. J. Agric. Sci. 127, 289–294.
Hue N V, Hirunburana H and Fox R L 1988 Boron status of Hawaiian USA soils as measured by boron sorption and plant uptake. Comm. Soil Sci. Plant Anal. 19, 517–528.
Jones C A, Inskeep WP and Neuman D R 1997 Arsenic transport in contaminated mine tailings following liming.J. Environ. Qual. 26, 433–439.
Jones K C, Davies B E and Peterson P J 1986 Silver in Welsh UK soils. Physical and chemical distribution studies. Geoderma 37, 157–174.
Jones K C and Peterson P J 1986 The influence of humic and fulvic acids on silver uptake by perennial ryegrass, and its relevance to the cycling of silver in soils. Plant Soil 95, 3–8.
Lehto T and Mälkönen E 1994 Effects of liming and boron fertilization on boron uptake of Picea abies. Plant Soil 163, 55–64.
Magdoff F R and Bartlett R J 1980 Effect of liming acid soils on potassium availability. Soil Sci. 129, 12–14.
Mandal B, Pal S and Mandal L N 1998 Effect of molybdenum, phosphorus and lime application to acid soils on dry matter yield and molybdenum nutrition of lentil. J. Plant Nutr. 21, 139–147.
McBride M B 1978 Transition metal bonding in humic acid. An ESR study. Soil Sci. 126, 200–209.
More E and Coppenet M 1980 Selenium contents of forage plants. Effects of fertilization and selenite application. Ann. Agron. (Paris) 31, 297–318.
Öborn I, Jansson G and Johnsson L 1995 A field study on the influence of soil pH on trace element levels in spring wheat (Triticum aestivum), potatoes (Solanum tuberosum and carrots (Daucus carota). Wat. Air Soil Pollut. 85, 835–840.
Paasikallio A 1979 Strontium content and strontium / calcium ratio in timothy, Phleum pratense, and soil in Finland. Ann. Agric. Fenn. 18, 174–181.
Preer J R, Abdi A N, Sekhon H S and Murchison G B 1995 Metals in urban gardens – effect of lime and sludge. J. Environ. Sci. A 30, 2041–2056.
Sadiq M 1997 Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Wat. Air Soil Pollut. 93, 117–136.
Sippola J 1980 Selenium content of soils and timothy, Phleum pratense, in Finland. Ann. Agric. Fenn.18(3).
Smith S R 1994 Effect of soil pH on availability to crops of metals in sewage sludge-treated soils. I. Nickel, copper and zinc uptake and toxicity to ryegrass. Environ. Pollut. 85, 321–327.
Tolgyesi G 1990 Boron content of Lucerne. Acta Agron. Hung. 39, 287–296.
Tyler G 1981 Leaching of metals from the A-horizon of a spruce forest soil. Wat. Air Soil Pollut. 15, 353–369.
Tyler G 1996 Soil chemistry and plant distributions in rock habitats of southern Sweden. Nord. J. Bot. 16, 609–635.
Tyler G 1997 Influence of acidity and potassium saturation on plant uptake of indigenous soil rubidium. Environ. Exp. Bot. 38, 181–186.
Tyler G 2000 Effects of sample pretreatment and sequential fractionation by centrifuge drainage on concentrations of minerals in a calcareous soil solution. Geoderma 94, 59–70.
Van Bergeijk K E, Noordijk H, Lembrechts J and Frissel MJ 1992 Influence of soil pH, soil type and soil organic matter content on soil-to-plant transfer of radiocesium and strontium as analyzed by a nonparametric method. J. Environ. Radioact. 15, 265–276.
Veresoglou D S, Barbayiannis N, Matsi T, Anagnostopoulos C and Zalidis G C 1996 Shoot Sr concentrations in relation to shoot Ca concentrations and to soil properties. Plant Soil 178, 95–100.
Wehrli B and Stumm W 1989 Vanadyl in natural waters. Adsorption and hydrolysis promote oxygenation. Geochim. Cosmochim.Acta 53, 69–78.
Zohlen A 2000 The use of 1,10-phenanthroline in estimating metabolically active Fe in plants. Comm. Soil Sci. Plant Anal. 31, 481–500.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tyler, G., Olsson, T. Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant and Soil 230, 307–321 (2001). https://doi.org/10.1023/A:1010314400976
Issue Date:
DOI: https://doi.org/10.1023/A:1010314400976