Abstract
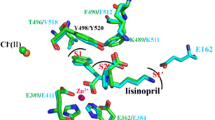
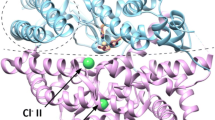
The interaction of three forms of bovine angiotensin-converting enzyme (ACE) with the competitive peptide inhibitor lisinopril with a fluorescent label was studied by the fluorescence polarization technique. The dissociation constants K d of the enzyme-inhibitor complexes in 50 mM Hepes-buffer, pH 7.5, containing 150 mM NaCl and 1 μM ZnCl2 at 37°C were (2.3 ± 0.4)·10−8, (2.1 ± 0.3)·10−8, and (2.1 ± 0.2)·10−8 M for two-domain somatic ACE, single-domain testicular ACE, and for the N-domain of the enzyme, respectively. The interaction of the enzyme with the inhibitor strongly depended on the presence of chloride in the medium, and the apparent dissociation constant of the ACE-chloride complex was (1.3 ± 0.2)·10−3 M for the somatic enzyme. The dissociation kinetics of the complex of the inhibitor with somatic ACE did not fit the kinetics of a first-order reaction, but it was approximated by a model of simultaneous dissociation of two complexes with the dissociation rate constants (0.13 ± 0.01) sec−1 and (0.026 ± 0.001) sec−1 that were present at approximately equal initial concentrations. The dissociation kinetics of the single-domain ACE complexes with the inhibitor were apparently first-order, and the dissociation rate constants were similar: (0.055 ± 0.001) and (0.041 ± 0.001) sec−1 for the N-domain and for testicular ACE, respectively.
Similar content being viewed by others
REFERENCES
Eliseeva, Yu. E. (1998) Bioorg. Khim., 24, 262–270.
Erdös, E. G., and Skidgel, R. A. (1987) Lab. Invest., 56, 345–348.
McAlpine, H. M., Morton, J. J., Leckie, B., Rumley, A., Gillen, G., and Dargie, H. J. (1988) Brit. Heart J., 60, 117–124.
Greenvald, L., and Becker, R. C. (1994) Am. Heart J., 128, 997–1009.
Soubrier, F., Alhehc-Gelas, F., Hubert, C., Allegrini, J., John, M., Tregear, G., and Corvol, P. (1988) Proc. Natl. Acad. Sci. USA, 85, 9386–9390.
Wei, L., Alhenc-Gelas, F., Corvol, P., and Clauser, E. (1991) J. Biol. Chem., 266, 9002–9008.
Ehlers, M. R., Fox, E. A., Strydom, D. J., and Riordan, J. F. (1989) Proc. Natl. Acad. Sci. USA, 86, 7741–7745.
Isaak, R. E., Williams, T. A., Sajid, M., Corvol, P., and Coates, D. (1997) Biochem. J., 328, 587–591.
Hagaman, J. R., Moyer, J. S., Bachman, E. S., Sibony, M., Magyar, P. L., Welch, J. E., Smithies, O., Krege, J. H., and O'Brien, D. A. (1998) Proc. Natl. Acad. Sci. USA, 95, 2552–2557.
Deddish, P. A., Wang, J., Michel, B., Morris, P. W., Davidson, N. O., Skidgel, R. A., and Erdös, E. G. (1994) Proc. Natl. Acad. Sci. USA, 91, 7807–7811.
Yotsumoto, H., Lanzillo, J. J., and Fanburg, B. L. (1983) Biochim. Biophys. Acta, 749, 180–184.
Sturrock, E. D., Danilov, S. M., and Riordan, J. F. (1997) Biochem. Biophys. Res. Commun., 236, 16–19.
Williams, T. A., Corvol, P., and Soubrier, F. (1994) J. Biol. Chem., 269, 29430–29434.
Jaspard, E., Wei, L., and Alhenc-Gelas, F. (1993) J. Biol. Chem., 268, 9496–9503.
Wei, L., Clauser, E., Alhenc-Gelas, F., and Corvol, P. (1992) J. Biol. Chem., 267, 13398–13405.
Rousseau, A., Michaud, A., Chauvet, M.-T., Lenfant, M., and Corvol, P. (1995) J. Biol. Chem., 270, 3656–3661.
Deddish, P. A., Wang, L.-X., Jackman, H. L., Michel, B., Wang, J., Skidgel, R. A., and Erdös, E. G. (1996) J. Pharmacol. Exp. Ther., 279, 1582–1589.
Deddish, P. A., Marcic, B., Jackman, H. L., Wang, H.-Z., Skidgel, R. A., and Erdös, E. G. (1998) Hypertension, 31, 912–917.
Ehlers, M. R., Chen, Y.-N. P., and Riordan, J. F. (1991) Protein Exp. Purif., 2, 1–9.
Binevski, P. V., Nikol'skaya, I. I., Pozdnev, V. F., and Kost, O. A. (2000) BiochemistryMoscow), 65, 651–658.
Grinshtein, S. V., Binevski, P. V., Gomazkov, O. A., Pozdnev, V. F., Nikol'skaya, I. I., and Kost, O. A. (1999) Biochemistry (Moscow), 64, 938–944.
Jameson, D. M., and Sawyer, W. (1995) Meth. Enzymol., 246, 283–300.
Dandliker, W. B., Kelly, R. J., Dandliker, J., Farquahar, J., and Levin, J. (1973) Immunochemistry, 10, 219–227.
Choi, M. J., Choi, J., Yoon, D. Y., Park, J., and Eremin, S. A. (1997) Biol. Pharm. Bull., 20, 309–314.
Checovich, W. J., Bolger, R. E., and Burke, T. (1995) Nature, 375, 254–256.
Colbert, D. L., Eremin, S. A., and Landon, J. (1991) J. Immunol. Meth., 140, 227–233.
Pourfarzanch, M., White, G. W., Landon, J., and Smith, D. S. (1982) Clin. Chem., 28, 1314–1318.
Bull, H. G., Thornberry, N. A., and Cordes, E. H. (1985) J. Biol. Chem., 206, 2963–2972.
Kost, O. A., Grinstein, S. V., Nikol'skaya, I. I., Shevchenko, A. A., and Binevski, P. V. (1997) Biochemistry (Moscow), 62, 321–328.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Ehlers, M. R., and Riordan, J. F. (1991) Biochemistry, 30, 7118–7126.
Keleti, T. (1990) Basic Enzyme Kinetics [Russian translation], Mir, Moscow, pp. 183–209.
Dixon, M., and Webb, E. C. (1982) Enzymes [Russian translation], Mir, Moscow, pp. 518–528.
Glaser, C. B., Brodrick, J. W., Dreshel, D., Karic, L., Graceffo, M., and Largman, C. (1982) Biochemistry, 21, 556–561.
Corvol, P., Williams, T. A., and Soubrier, F. (1995) Meth. Enzymol., 248, 283–305.
Shapiro, R., Holmquist, B., and Riordan, J. F. (1983) Biochemistry, 22, 3850–3857.
Martin, M., Vallee, B. L., and Riordan, J. F. (1987) Anal. Biochem., 161, 341–347.
Gutierrez, M. C., Gomez-Hens, A., and Perez-Bendito, D. (1989) Talanta, 36, 1187–1201.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Voronov, S.V., Binevski, P.V., Eremin, S.A. et al. Fluorescence Polarization Studies of Different Forms of Angiotensin-Converting Enzyme. Biochemistry (Moscow) 66, 788–794 (2001). https://doi.org/10.1023/A:1010220930765
Issue Date:
DOI: https://doi.org/10.1023/A:1010220930765