Abstract
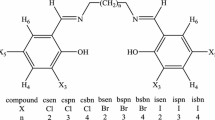
Potentiometric studies on Schiff bases derived from5,7-dihydroxy-6-formyl-2-methylbenzopyran- 4-one were carried out at different ionic strengths (0.02, 0.04, 0.06, 0.10 and 0.14 M NaCl), at different temperatures (25, 35, 45 and55°C) and in ethanol-water media of varying compositions (60, 70, 80 and 90% v/v). The ionization constants of the Schiff bases were investigated in the presence of different organic solvents (70% v/v), e.g. methanol, ethanol, n-propanol, isopropanol, acetone, DMSO and DMF-water media. The thermodynamic parameters (ΔG, ΔH and ΔS) were also calculated.
Similar content being viewed by others
References
C. R. Bera, S. Chattpadhyay and G. P. Sengupta, J. Indian Chem. Soc., 56 (1979) 416.
M. S. Masoud and F. M. El-Zawawy, Indian J. Chem., 23A (1984) 149.
T. Gunduz and K. Esma, Analyst (London), 112 (1987) 1057.
T. Gunduz, E. Kilic, E. Canel and F. Koeseoglu, Anal. Chem. Acta, 282 (1993) 489.
J. Bjerrum, 'Metal-Amine Formation in Aqueous Solution', Haase, Copenhagen 1941.
M. Calvin and K. W. Wilson, J. Am. Chem. Soc., 67 (1945) 2003.
H. M. Irving and H. S. Rossotti, J. Chem. Soc., (1945) 2904.
A. Vogel, 'Practical Organic Chemistry Including Quantitative Organic Analysis', 5th Ed., Longmans, London 1991.
A. A. Abdel-Gaber, A. M. A. N. Hassaan, M. El-Shabasy and A. M. El-Roudi, Synth. React. Inorg. Met. Org. Chem., 21 (1991) 1265.
M. El-Roudi, Bull Fac. Sci. Assuit University, 18 (1989) 77.
P. K. Jadhav, T. D. Mathews and P. V. Kamat, J. Indian Chem. Soc., 63 (1986) 894.
K. Denbigh, 'Principles of Chemical Equilibrium', Cambridge Univ. Press, London 1955.
S. Mukherjee and N. S. Rawat, J. Indian Chem. Soc., 56 (1979) 413.
A. A. El-Bindary, Monatsh. Chem., 125 (1994) 811.
S. A. Shama, Egypt. J. Anal. Chem., 5 (1996) 13.
F. R. Hartley, 'Solution Equilibria', John Wiley and Sons, New York 1980.
O. E. Sherif and S. M. Abass, Commun. Fac. Sci. Univ. Ank. Series B, 39 (1993) 37.
G. Czharter and B. Temillon, 'Chemical Reactions in Solvents and Melts' Pergamon Press, London 1969.
N. T. Abdel-Ghani and Z. Z. Sharara, Egypt. J. Chem., 32 (1989) 533.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sherif, O.E., Issa, Y.M. & Abbas, S.M. Thermodynamic Parameters of Some Schiff Bases Derived From 5,7-dihydroxy-6-formyl-2-methylbenzopyran-4-one. Journal of Thermal Analysis and Calorimetry 59, 913–926 (2000). https://doi.org/10.1023/A:1010182527146
Issue Date:
DOI: https://doi.org/10.1023/A:1010182527146