Abstract
The freezing and melting of water in semi-dilute (0.5–3.0%) solutions of the polysaccharide hyaluronanhave been investigated by modulated differential scanning calorimetry.
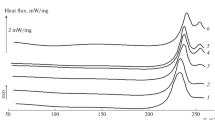
High molecular weight hyaluronan inhibited nucleation of ice and significantly depressed thefreezing temperature in a dynamic scan conducted at −3.0°C min−1. Low molecular weight hyaluronan had a weaker and more variable effect on nucleation. Theeffects on nucleation, especially by the high molecular weight hyaluronan, are attributed tothe influence of a hyaluronan network on the formation of critical ice nuclei.
Both high and low molecular weight hyaluronan reduced the melting temperature of ice by 0.4–1.1°C, depending on concentration. The enthalpy change associated with this transitionwas significantly reduced. If all of the enthalpy difference is attributed to the presence of non-freezing water, approximately 3.65 g water/g hyaluronan would be non-freezing. This result appears incompatible with published studies on hyaluronan samples of low water content. An alternative hypothesis and quantitative approach to analysis of the data are suggested. The data are interpreted in terms of a small amount of non-freezing water, and amuch larger boundary layer of water surrounding hyaluronan chains, which has slightly altered thermodynamic properties relative to those of bulk water. The boundary layer water behaves similarly to water trapped in small pores in solid materials and hydrogels.
Similar content being viewed by others
References
W. D. Comper and T. C. Laurent, Physiol. Rev., 58 (1978) 255.
F. A. Meyer, Biochim. Biophys. Acta, 755 (1983) 388.
T. C. Laurent, ‘Chemistry and Molecular Biology of the Extracellular Matrix’, E. A. Balazs, Ed., Academic Press, New York 1970, pp. 703-732.
T. C. Laurent, Biophys. Chem., 57 (1995) 7.
E. A. Balazs, Fed. Proc., 25 (1966) 1817.
D. A. Gibbs, E. W. Merrill, K. A. Smith and E. A. Balazs, Biopolymers, 6 (1968) 777.
Y. Suzuki and H. Uedaira, Bull. Chem. Soc. Jpn., 43 (1970) 1892.
A. Davies, J. Gormally, E. Wyn-Jones, D. J. Wedlock and G. O. Phillips, Biochem. J., 213 (1983) 363.
H. Yoshida, T. Hatakeyama and H. Hatakeyama, ‘Cellulose’, J. F. Kennedy, G. O. Phillips, P. A. Williams, Eds., Horwood, Chichester, UK 1990, pp. 305-310.
H. N. Joshi and E. M. Topp, Int. J. Pharm., 80 (1992) 213.
H. Yoshida, T. Hatakeyama and H. Hatakeyama, J. Thermal Anal., 40 (1993) 483.
N. Jouon, M. Rinaudo, M. Milas and J. Desbrières, Carbohydr. Polym., 26 (1995) 69.
L. Picullel, B. Lindman and R. Einarsson, Biopolymers, 23 (1984) 1683.
C. R. Ethier, Biorheology, 23 (1986) 99.
H. D. Middendorf, D. Di Cola, F. Cavatorta, A. Deriu and C. J. Carlile, Biophys. Chem., 53 (1994) 145.
F. A. Bettelheim and N. Popdimirova, Curr. Eye Res., 11 (1992) 411.
H. G. Lee and M. K. Cowman, Anal. Biochem., 219 (1994) 278.
H. Bothner, T. Waaler and O. Wik, Int. J. Biol. Macromol., 10 (1988) 287.
E. A. Balazs, ‘The Amino Sugars’, Vol. 2A, E. A. Balazs and R. W. Jeanloz, Eds., Academic Press, New York 1965, pp. 401-460.
M. K. Cowman, J. Liu, M. Li, D. M. Hittner and J. S. Kim, ‘The Chemistry, Biology, and Medical Applications of Hyaluronan and its Derivatives’, T. C. Laurent, Ed., Portland Press, London 1998, pp. 17-24.
Y. P. Handa, M. Zakrzewski and C. Fairbridge, J. Phys. Chem., 96 (1992) 8594.
K. F. Arndt and P. Zander, Colloid Polym. Sci., 268 (1990) 806.
R. L. Cleland, Biopolymers, 18 (1979) 2673.
J. C. Benegas, A. Di Blas, S. Paoletti and A. Cesàro, J. Thermal Anal., 38 (1992) 2613.
F. X. Quinn, E. Kampff, G. Smyth and V. J. McBrierty, Macromol., 21 (1988) 3191.
T. W. Schenz, B. Israel and M.A. Rosolen, ‘Water Relationships in Foods’, H. Levine and L. Slade, Eds., Plenum Press, New York 1991, pp. 199-214.
S. Takigami, M. Takigami and G. O. Phillips, Carbohydr. Polym., 22 (1993) 153.
L. Bosio, G. P. Johari, M. Oumezzine and J. Teixeira, Chem. Phys. Lett., 188 (1992) 113.
A. Davies, J. Gormally, E. Wyn-Jones, D.J. Wedlock and G. O. Phillips, Int. J. Biol. Macromol., 4 (1982) 436.
M. K. Cowman, M. Li and E. A. Balazs, Biophys. J., 75 (1998) 2030.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Cowman, M.K. Thermal Analysis of Semi-Dilute Hyaluronan Solutions. Journal of Thermal Analysis and Calorimetry 59, 547–557 (2000). https://doi.org/10.1023/A:1010114213475
Issue Date:
DOI: https://doi.org/10.1023/A:1010114213475