Abstract
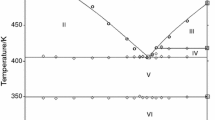
Phase diagram of the binary system CsNO3−LiNO3 has been drawn by using simultaneously direct and differential thermal analysis between 323 and 723 K. This system is characterized by a congruent intermediate equimolar compound with melting point at 463 K, two eutectic reactions at 447 and 433 K; the eutectic points are respectively at 0,47 and 0,63 mol fraction of LiNO3; a plateau due to the phase transition of CsNO3 at 428 K and an other one at 333 K due to the formation of CsLi(NO3)2. The miscibility in solid state seems to be nil or negligible. These results associated with some other thermodynamic data have been used to calculate the activities of the constituents along the liquidus curve and the activities of the liquid constituents at 723 K. The binary liquid (Cs−Li)NO3 exhibits a negative deviation from the ideal behaviour.
Similar content being viewed by others
Références
N. Belaï d-Drira, H. Zamali et M. Jemal, XXII suè mes Journées d.Etudes des Equilibres entre Phases; 15-16 Avril 1996, La Garde-Toulon, France, 137, 1996.
N. A. Puschin and M. Radonicic, Z. Anorg. Allgem. Chem., 233 (1937) 41.
V. P. Blidin, Izv. Sekt. Fiz. Khim. Anal., Inst. Obsch. Neorg. Khim. Akad. Nauk SSSR, 23 (1953) 233.
P. I. Protsenko and T. I. Fedchenko, Uch. Zap. Rostov-Na-Donu. Gos. Univ., 60 (1959) 135.
N. N. Nurminskii and G. G. Diogenov, Russ. J. Inorg. Chem., 5 (1960) 1011.
K. A. Bol'shakov, B. I. Pokrovskii and V. E. Plyushchev, Zh. Neorg. Khim., 6 (1961) 2120.
P. I. Protsenko, R. P. Shisholina and E. M. Ivanova, Izv. Vyssh. Uchebn. Zaved. Khim. I. Khim. Nauk SSSR, 7 (1964) 180.
G. Litvinov, Yu. I. I. Il'yasov and V. I. Savva, Zh. Neorg. Khim., 20 (1975) 2560.
O. J. Kleppa and L. S. Hersh, J. Chem. Phys., 34 (1961) 351.
H. Zamali, Thè se de Doctorat de spécialité, option chimie, Fac. Sc. Tunis, 1982.
H. Zamali et M. Jemal, Journées de Montpellier, AFCAT, 20-22 Mai, 16 (1985) 298.
H. Zamali and M. Jemal, J. Thermal Anal., 41 (1994) 1.
H. Zamali, Thè se de Doctorat d'Etat en chimie, N° D 194, Fac. Sc. Tunis, 1996.
N. Belaï d-Drira, H. Zamali et M. Jemal, J. Thermal Anal., 46 (1996) 1449.
T. Jriri, Thè se de Doctorat de l'Académie d'Aix-Marseille, 1994.
B. B. Ownes, J. Chem. Phys., 42 (1965) 2259.
Y. Dessureault, J. Sangster et A. D. Pelton, J. Chim. Phys., 87 (1990) 407.
N. Mossarello, Thè se d'Université de Provence, ‘Aix-Marseille I’, 1986.
K. Ichikawa and T. Matsumoto, Bull. Chem. Soc. Japan, 56 (1983) 2093.
R. Connan, Rev. Soc. Chim. Mex., 22 (1978) 17.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bélaïd-Drira, N., Zamali, H. & Jemal, M. Diagramme De Phases Du Systeme Binaire CsNO3—LiNO3. Activité des constituants du liquide. Journal of Thermal Analysis and Calorimetry 58, 607–615 (1999). https://doi.org/10.1023/A:1010104528126
Issue Date:
DOI: https://doi.org/10.1023/A:1010104528126