Abstract
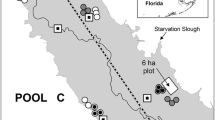
Over the last century, Phragmites australis (common reed) has been expanding rapidly from the marsh–upland boundary into Spartina patens (salt hay)-dominated high marsh communities of the eastern US coast. Whereas direct and indirect human disturbances and changes in hydrology or salinity are likely to influence rates of spread at the landscape scale, the susceptibility of specific plant communities to invasion also influence rates of Phragmites expansion at the local scale. I measured microscale (0.25 m2) spatial patterns of culms (emerging buds and mature stems) in October 1993 at both expanding and stable boundaries of Phragmites populations within a S. patens-dominant matrix. In both expanding and stable plots, Phragmites culms were observed more frequently than expected on hummocks that were created by S. patens tussock-forming root structure. Culm density within a plot was correlated with the percent hummock cover within a plot. Further, Phragmites culms, particularly mature stems, were concentrated along the perimeter of the hummocks. Because the culms were not evenly distributed between hummocks and hollows, I suggest that invasion rates of Phragmites are limited in S. patens communities by microscale differences in hummock availability. The pattern of emergence suggests that expanding rhizomes of Phragmites encounter both competition with S. patens roots on the hummocks and physiological stressors (salinity, anoxia, sulfide concentrations) in the hollows.
Similar content being viewed by others
References
Bertness MD (1991) Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72(1): 125–137
Bertness MD and Ellison AM (1987) Determinants of pattern in a New England salt marsh community. Ecological Monographs 57(1): 129–147
Brix H (1989) Gas exchange through dead culms of reed, Phragmites australis (Cav.) Trin. Ex Steudel. Aquatic Botany 35: 81–98
Chambers R (1997) Porewater chemistry associated with Phragmites and Spartina in a Connecticut tidal marsh. Wetlands 17: 360–367
Durand JR (1988) Field Studies in the Mullica River-Great Bay Estuarine System. Volume 1. Rutgers University, Center for Coastal and Environmental Studies, 208 pp
Ekstam B (1995) Ramet size equalisation in a clonal plant, Phragmites australis. Oecologia 104: 440–446
Ferren WR, Good RE, Walker R and Arsenault J (1981) Vegetation and flora of Hog Island, a brackish wetland in the Mullica River, New Jersey. Bartonia 48: 1–10
Grace JB and Wetzel RG (1981) Habitat partitioning and competitive displacement in cattails (Typha): Experimental field studies. American Naturalist 118(4): 463–474
Harper JL (1977) Population Biology of Plants. Academic Press, London
Haslam SM (1971) Community regulation in Phragmites communis Trin. II. Mixed stands. Journal of Ecology 59: 75–87
Haslam SM (1972) Biological flora of the British Isles: Phragmites communis Trin. Journal of Ecology 60: 585–610
Havens KJ, Priest WI and Berquest H (1997) Investigation and long term monitoring of Phragmites australis within Virginia's constructed wetland sites. Environmental Management 21: 599–605
Hellings SE and Gallagher JL (1992) The effects of salinity and flooding on Phragmites australis. Journal of Applied Ecology 29: 41–49
Higgins SI, Richardson DM and Cowling RM 1996. Modeling invasive plant spread: The role of plant-environment interactions and model structure. Ecology 77: 2043–2054
Huber-Sannwald E, Pyke DA, Caldwell MM and Durham S (1998) Effects of nutrient patches and root systems on the clonal plasticity of a rhizomatous grass. Ecology 79: 2267–2280
Levine J, Brewer S and Bertness MD (1998) Nutrient availability and the zonation of marsh plant communities. Journal of Ecology 86: 285–292
Miller WR and Egler FE (1950) Vegetation of the Wequetequock-Pawcatuck tidal marshes, Connecticut. Ecological Monographs 20: 143–172
Niering WA and Warren RS (1980) Vegetation patterns and processes in New England salt marshes. Bioscience 30: 301–307
Orson RA, Warren RS and Niering WA (1987) Development of a tidal marsh in a New England river valley. Estuaries 10: 20–27
Salesman AG and Parker MA (1985) Neighbors ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia 65: 273–277
Sauer J (1988) Plant Migration: The Dynamics of Geographic Patterning in Seed Plant Species. University of California Press, Berkeley, California
Silvertown J (1982) Introduction to Plant Population Ecology, Longman, New York
Sokal RR and Rohlf FJ (1978) Biometry. W.H. Freeman and Co, San Francisco, California
Westerfelt J, Goran W and Shapiro M (1987) Design and development of GRASS: The Geographic Resources Analysis Support System, U.S. Army Construction Engineering Research Laboratory. Champaign, Illinois
Windham L (1995) Effects of Phragmites australis on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. MS thesis. Rutgers University, New Jersey
Windham L and Lathrop R (1999) Effects of Phragmites australis on aboveground biomass and soil properties in brackish tidal marsh. Estuaries 22(4): 927–935
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Windham, L. Microscale Spatial Distribution of Phragmites australis (Common Reed) Invasion into Spartina patens (Salt Hay)-dominated Communities in Brackish Tidal Marsh. Biological Invasions 1, 137–148 (1999). https://doi.org/10.1023/A:1010016319074
Issue Date:
DOI: https://doi.org/10.1023/A:1010016319074