Abstract
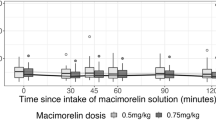
GH secretagogues (GHS) possess potent GH-releasing activity but also stimulate PRL, ACTH and cortisol (F) secretion. To further clarify the endocrine activities of GHS, in 9 obese patients, 9 patients with Cushing's Disease and 14 controls we studied the ACTH, F, GH and PRL responses to hexarelin (HEX, 2.0 µg/kg i.v.), a peptidyl GHS, alone and preceeded by alprazolam (ALP, 0.02 mg/kg p.o.), a benzodiazepine. The HEX-induced ACTH response in controls was similar to that in obese patients (Δpeak: 9.9 ± 1.9 and 24.7 ± 7.6 ng/L, respectively) and both were lower (p < 0.002) than that in Cushing's patients (peak: 210.7 ± 58.4 ng/L). The GH response to HEX in controls (peak: 58.1 ± 10.3 □g/L) was higher (p < 0.001) than those in obese and Cushing's patients (18.2 ± 3.8 and 12.6 ± 5.4 □/L, respectively) which, in turn, were similar. The PRL responses to HEX in controls, obese and Cushing's patients (peak: 11.9 ± 1.6, 18.0 ± 4.5 and 12.4 ± 1.4 □/L, respectively) were similar. In controls the HEX-induced ACTH response was abolished by ALP (peak: 8.6 ± 2.4 vs 28.0 ± 6.7 ng/L, p < 0.03) which, on the other hand, only blunted that in obese (peak: 12.7 ± 2.1 vs 42.4 ± 8.4 ng/L, p < 0.02) and did not modify that in Cushing's patients (205.6 ± 55.4 vs 175.9 ± 47.6 ng/L). ALP blunted the GH response to HEX in controls (peak: 31.0 ± 7.1 □/L, p < 0.03) while did not modify those in obese and in Cushing's patients (14.5 ± 5.3 and 13.3 ± 11.1 □/L, respectively). ALP did not modify the HEX-induced PRL response in controls, obese and Cushing's patients (peak: 13.8 ± 0.9, 16.3 ± 2.4 and 19.2 ± 1.1 □/L, respectively). In conclusion, alprazolam inhibits the ACTH response to hexarelin in normal and obese subjects while fails to modify the exaggerated ACTH response in Cushing's Disease suggesting that GHS activate the HPA axis via the hypothalamus in normal and obese subjects but not in patients with Cushing's disease. Alprazolam is also able to blunt the GH-releasing activity of hexarelin in normal subjects but not the low GH response to the hexapeptide in obese and Cushing's patients. The PRL-releasing activity of hexarelin in controls, obese and hypercortisolemic patients is similar and is not modified by alprazolam pretreatment.
Similar content being viewed by others
References
Bowers CY, Veeraragavan K, Sethumadhavan K. Atypical growth hormone releasing peptides. In: Bercu BB, Walker RF (eds). Growth Hormone II, Basic and Clinical Aspects. New York: Springer 1993;203–222.
Ghigo E, Arvat E, Muccioli G, Camanni F. Growth hormone (GH)-releasing peptides. Eur J Endocrinol 1997;136:445–460.
Smith RG, Van der Ploeg LXT, Howard AD, Feighner SD, Cheng K, Hickey GJ, WyvrattMJ, Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev 1997;18:621–645.
Deghenghi R, Boutignon F, Luoni M, Grilli R, Guidi M, Locatelli V. Small peptides as potent releasers of growth hormone. J Ped Endocrinol Metab 1995;8:311–313.
Jacks T, Hickey G, Judith F, Taylor J, Chen H, Krupa D, Freeney W, Schoen W, Fisher M, Wyratt M, Smith R. Effects of acute and repeated intravenous administration of L-692,585, a novel non-peptidyl growth hormone secretagogue, on plasma growth hormone, IGF-I, ACTH, cortisol, prolactin, insulin and thyroxine levels in beagles. J Endocrinol 1994;143:399–406.
Hickey GJ, Drisko J, Faidley T, Chang C, Anderson L, Nicolich S, McGuire L, Riches E, Krupe D, FeeneyW, Friscino R, Cunninghan P, Frazier E, Chen H, Leroque P, Smith RG. Mediation by the central nervous system is critical to the in vivo activity of the GH secretagogue L-692,585. J Endocrinol 1996;148:371–380.
Thomas GB, Fairhall KM, Robinson ICAF. Activation of the hypothalamo-pituitary-adrenal axis by the growth hormone (GH) secretagogue, GH releasing peptide-6, in rats. Endocrinology 1997;138:1585–1591.
Korbonits M, Trainer PJ, Besser GM. The effect of an opiate antagonist on the hormonal changes induced by hexarelin. Clin Endocrinol 1995;43:365–371.
Arvat E, Ramunni J, Bellone J, Di Vito L, Baffoni C, Broglio F, Deghenghi R, Bartolotta E, Ghigo E. The GH, prolactin, ACTH and cortisol responses to hexarelin, a synthetic hexapeptide, undergo different age-related variations. Eur J Endocrinol 1997;137:635–642.
Ghigo E, Arvat E, Ramunni J, Colao A, Gianotti L, Deghenghi R, Lombardi G, Camanni F. Adrenocorticotropin-and cortisol-releasing effect of hexarelin, a synthetic growth hormone-releasing peptide, in normal subjects and patients with Cushing's syndrome. J Clin Endocrinol Metab 1997;82: 2439–2444.
Arvat E, Di Vito L, Maccagno B, Broglio F, Boghen MF, Deghenghi R, Camanni F, Ghigo E. Effects of GHRP-2 and Hexarelin, two synthetic releasing peptides, on GH, prolactin, ACTH and cortisol levels in man. Comparison with the effects of GHRH, TRH and hCRH. Peptides 1997;18: 885–891.
Arvat E, Maccagno B, Ramunni J, Di Vito L, Broglio F, Deghenghi R, Camanni F, Ghigo E. Hexarelin, a synthetic growth-hormone releasing peptide, shows no interaction with corticotropin-releasing hormone and vasopressin on adrenocorticotropin and cortisol secretion humans. Neuroendocrinology 1997;66:432–438.
Codd EE, Shu AYL, Walker RF. Binding of a growth hormone releasing hexapeptide to specific hypothalamic and pituitary binding sites. Neuropharmacology 1989;28:1139–1144.
Pong SS, Chaung LYP, Dean DC, Nargunt RP, Patchett AA, Smith RG. Identification of a new G-protein-linked receptor for a growth hormone secretagogues. Mol Endocrinol 1996; 10:57–61.
Howard AD, Heighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Uamelin M, Ureniuk DL, Palyhe OC, Anderson J, Paress PS, Diaz C, Chou N, Liu KK, McKee KK, Pong SS, Cheung LY, Elbrecht A, Daskevitcz M, Keavehs R, Rishy M, Sirinjhsinghji DJS, Dean DC, Melillo DG, Patchett AA, Nergund R, Srillin PR. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996;273:974–977.
Ong H, McNicoll N, Esher E, Collu R, Deghenghi R, Locatelli V, Ghigo E, Muccioli G, Boghen M, Nillson MHL. Identification of a pituitary GHRP receptor subtype by the photoaffinity labeling approach using [125-I]p-benzoylphenylalanine Hexarelin derivative. Endocrinology 1998; 139:432–435.
Muccioli G, Ghè C, Ghigo MC, Papotti M, Arvat E, Boghen MF, Nilsson MH, Deghenghi R, Ong H, Ghigo E. Specific receptos for synthetic GH secretagogues in the human brain and pituitary gland. J Endocrinol 1998;157:99–106.
McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LHT. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 1997;46:426–434.
Goth MI, Lyons CE, Canny BY, Thorner MO. Pituitary adenylate cyclase acting polypeptide, growth hormone (GH)-releasing peptide and GH-releasing hormone stimulate GH release through distinct pituitary receptors. Endocrinology 1992;130:939–944.
Fairhall KM, Mynett A, Robinson ICAF. Central effects of growth hormone-releasing hexapeptide (GHRP-6) on growth hormone release are inhibited by central somatostatin action. J Endocrinol 1995;144:555–560.
Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology 1997;13:771–777.
Bennet PA, Thomas GB, Howard AD, Feigher SD, Van der Ploeg LHT, Smith RG, Robinson ICAF. Hypothalamic growth hormone secretagogue-receptor (GHS-R) expression is regulated by growth hormone in the rat. Endocrinology 1997;138:4552–4557.
Popovic V, Damjanovic S, Micic D, Djurovic M, Dieguez C, Casanueva FF. Blocked growth hormone-releasing peptide (GHRP-6)-induced GH secretion and absence of the synergic action of GHRP-6 plus GH-releasing hormone in patients with hypothalamic-pituitary disconnection: Evidence that GHRP-6 main action is exerted at the hypothalamic level. J Clin Endocrinol Metab 1995;80:942–947.
Arvat E, Gianotti L, Di Vito L, Imbimbo BP, Lenaerts V, Deghenghi R, Ghigo E. Modulation of the growth hormone releasing activity of hexarelin in man. Neuroendocrinology 1995;61:51–56.
Renner U, Brockmeier S, Strasburger CJ, Lange M, Schopohl J, Muller OA. Growth hormone (GH)-releasing peptide stimulation of GH release from human somatotroph adenoma cells: Interaction with GH-releasing hormone, thyrotropin-releasing hormone and octreotide. J Clin Endocrinol Metab 1994;78:1090–1096.
Adams EF, Petersen B, Lei T, Buchfelder M, Fahlbush R. The growth hormone secretagogue, L-692,429, induces phosphatidylinositol hydrolysis and hormone secretion by human pituitary tumors. Biochemical and Biophysical Research Communications 1995;208:555–561.
Shimon I, Yan Xinmin, Melmed S. Human fetal pituitary expresses functional growth hormone-releasing peptide receptors. J Clin Endocrinol Metab 1998;83:174–178.
Risby ED, Hsiao JK, Golden RN, Potter WZ. Intravenous alprazolam challange in normal subjects, biochemical, cardiovascular and behavioural effects. Psychopharmacology 1989;99:508–514.
Zemishlany Z, McQueeney R, Gabriel SM, Davidson M. Neuroendocrine and monoaminergic responses to acute administration of alprazolam in normal subjects. Neuropsychobiology 1990;23:124–128.
Kalogeras KT, Calogero AE, Kuribayashi T. In vitro and in vivo effects of the triazolobenzodiazepine alprazolam on hypothalamic pituitary-adrenal function: Pharmacologic and clinical implications. J Clin Endocrinol Metab 1990;70:1462–1471.
Torpy DJ, Grice EJ, Hockings GI, Walters MM, Crosbie GV, Jackson RV. Alprazolam blocks the naloxone-stimulated hypothalamo-pituitary-adrenal axis in man. J Clin Endocrinol Metab 1993;76:388–391.
Torpy DJ, Grice EJ, Hockings GI, Walters MM, Crosbie GV, Jackson RV. Alprazolam attenuates vasopressin-stimulated adrenocorticotropin and cortisol release: Evidence for synergy between vasopressin and corticotropin-releasing hormone in humans. J Clin Endocrinol Metab 1994;79:140–144.
Rohrer T, von Richthofen V, Schulz C, Beyer J, Lenhert H. The stress-, but not corticotropin-releasing hormone-induced activation of the pituitary-adrenal axis in man is blocked by alprazolam. Horm Metab Res 1994;26:200–206.
Breier A, Davis O, Buchanan R, Listwak SJ, Holmes C, Pickar D, Goldstein DS. Effects of alprazolam on pituitaryadrenal and catecholaminergic responses to metabolic stress in humans. Biol Psychiatry 1992;32:880–890.
Arvat E, Maccagno B, Ramunni J, Di Vito L, Gianotti L, Broglio F, Benso A, Deghenghi R, Camanni F, Ghigo E. Effects of dexamethasone and alprazolam, a benzodiazepine, on the stimulatory effect of hexarelin, a syntethic GHRP, on ACTH, cortisol and GH secretion in humans. Neuroendocrinology 1998;67:310–316.
Korbonits M, Trainer PJ, Edwards R, Besser JM, Grossman AB. Benzodiazepines attenuate the pituitary-adrenal responses to corticotropin-releasing hormone in healthy volunteers but not in patients with Cushing's syndrome. Clin Endocrinol 1995;43:29–35.
Eriksson E, Carlsson M, Nilsson C, Soderpalm B. Does alprazolam, in contrast to diazepam, activate alpha-2-adrenoceptors involved in the regulation of rat growth hormone secretion. Life Sci 1986;39:1491–1498.
Orth DN. Corticotropin-releasing hormone in humans. Endocr Rev 1992;13:164–191.
Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi F, Flamia R, Morselli-Labate AM, Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab 1993;77:341–346.
Pasquali R, Anconetani B, Chattat R, Biscotti M, Spinucci G, Casimirri F, Vicennati V, Carcello A, Morselli-Labate AM. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: Effects of corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism 1996;45:351–356.
Veldhuis JD, Iranmanesh A, Ho KKY, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab 1991;72:51–59.
Smith SR. The Endocrinology of obesity. Endocrinol Metab Clinics of North America 1996;25:921–942.
Arvat E, Giordano R, Ramunni J, Arnaldi G, Colao A, Deghenghi R, Lombardi G, Mantero F, Camanni F, Ghigo E. The ACTH and cortisol hyper-responsiveness to hexarelin, a peptidyl GH secretagogue (GHS), is not present in all patients with Cushing's disease. J Clin Endocrinol Metab (in press).
Procopio M, Invitti C, Maccario M, Grottoli S, Cavagnini F, Camanni F, Ghigo E. Effect of arginine and pyridostigmine on the GHRH-induced GH rise in obesity and in Cushing's syndrome. Int J Obesity 1995;19:108–112.
Cordido F, Penalva A, Dieguez C, Casanueva FF. Massive growth hormone (GH) discharge in obese subjects after combined administration of GH-releasing hormone and GHRP-6: Evidence for marked somatotroph secretory capability in obesity. J Clin Endocrinol Metab 1993;76:819–823.
Leal Cerro A, Pumar A, Garcia-Garcia E, Dieguez C, Casanueva FF. Inhibition of growth hormone release after the combined administration of GHRP and GHRP-6 in patients with Cushing's syndrome. Clin Endocrinol 1994;41:649–654.
Weaver JU, Kopelman PG, McLoughlin L, Forsling MI, Grossman AB. Hyperactivity of the hypothalamic-pituitaryadrenal axis in obesity: A study of ACTH, AVP, B-lipotropin and cortisol responses to insulin-induced hypoglycemia. Clin Endocrinol 1993;39:345–350.
Fletcher TP, Thomas GB, Willoughby JO, Clarke IJ. Constitutive growth hormone secretion in sheep after hypothalamo-pituitary disconnection and the direct in vivo pituitary effect of growth hormone releasing peptide 6. Neuroendocrinology 1994;60:76–86.
Elias KA, Ingle GS, Burnier JP, Hammonds RG, McDowell RS, Rawson TE, Somers TC, Stanley MS, Cronin MJ. In vitro characterization of four novel classes of growth hormone releasing peptide. Endocrinology 1995;136:5694–5699.
De Keyzer Y, Lenne F, Bertagna X. Widespread transcription of the growth hormone-releasing peptide receptor gene in neuroendocrine human tumors. Eur J Endocrinol 1997; 137:715–718.
Jansson JO, Svensson J, Bengtsson BA, Frohman LA, Ahlman H, Wanberg B, Nilsson O, Nilsson M. Acromegaly and Cushing's syndrome due to ectopic production of GHRHand ACTH by a thymic carcinoid tumour: In vitro responses to GHRH and GHRP-6. Clin Endocrinol 1998;48:243–250.
Korbonits M, Jacobs RA, Aylwin SJB, Burrin JM, Dahia PLM, Monson JP, Honegger J, Fahlbush R, Trainer PJ, Chew SL, Besser GM, Grossman AB. Expression of the growth hormone secretagogue receptor in pituitary adenomas and other neuroendocrine tumors. J Clin Endocrinol Metab 1998;83:3624–3630.
Holsboer F, Barden N. Antidepressants and hypothalamicpituitary-adrenocortical regulation. Endocr Rev 1996;17: 187–205.
Tannenbaum GS, Lapointe M, Gurd W, Finkelstein JA. Mechanisms of impaired growth hormone secretion in genetically obese Zucker rats: Roles of growth hormone-releasing factor and somatostatin. Endocrinology 1990;127: 3087–3095.
Peino R, Cordido F, Penalva A, Alvarez CV, Dieguez C, Casanueva FF. Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. J Clin Endocrinol Metab 1996;81: 909–913.
Maccario M, Procopio M, Grottoli S, Oleandri SE, Boffano GM, Taliano M. Effects of acipimox, an antilypolitic drug, on the growth hormone (GH) response to GH-releasing hormone alone or combined with arginine in obesity. Metabolism 1996;45:342–346.
Grottoli S, Maccario M, Procopio M, Oleandri SE, Arvat E, Gianotti L, Deghenghi R, Camanni F, Ghigo E. Somatotrope responsiveness to Hexarelin, synthetic hexapeptide, is refractory to the inhibitory effect of glucose in obesity. Eur J Endocrinol 1997;135:678–682.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grottoli, S., Arvat, E., Gauna, C. et al. Effects of Alprazolam, a Benzodiazepine, on the ACTH-, GH- and PRL-Releasing Activity of Hexarelin, a Synthetic Peptidyl GH Secretagogue (GHS), in Patients with Simple Obesity and in Patients with Cushing's Disease. Pituitary 2, 197–204 (1999). https://doi.org/10.1023/A:1009992909247
Issue Date:
DOI: https://doi.org/10.1023/A:1009992909247