Abstract
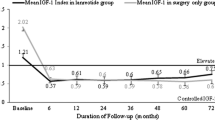
A European multicentre, open-label 12-month study with Sandostatin® LAR® administered intramuscularly at 4-week intervals was initiated in 151 acromegalics responsive to octreotide. All patients received 3 injections of the 20 mg dose, following which the dose was adjusted to 10 mg in patients with mean 4-hour GH serum concentrations below 1 µg/L (N: 29) and to 30 mg in patients with concentrations above 5 µg/L (N: 22). The GH level suppression was significant in the 20 mg dose group (p<0.01) and for all 151 patients (p<0.004), and was consistently maintained in all patients for the duration of the study. The suppression of the mean serum GH concentration to below 2.5 µg/L was recorded in 69.8% of patients at the endpoint treatment with Sandostatin® LAR® and 65.8% during prior treatment with Sandostatin® SC. A consistent suppression of serum IGF-I levels was also achieved. The number of patients with headache, fatigue, perspiration, joint pains and paresthesias had decreased significantly (p<0.05) after the 6th injection of Sandostatin® LAR® vs. previous SC treatment. No patient discontinued the study because of drug-related adverse events. The most frequently reported adverse events were mild diarrhea, abdominal pain and flatulence. The local tolerability was very good. No impairment of safety hematology, biochemistry and thyroid function tests and no increased incidence of gallstone formation was recorded. Well tolerated and at least as efficacious as the SC formulation, Sandostatin® LAR® might become an alternative primary treatment to pituitary surgery and radiotherapy.
Similar content being viewed by others
References
Wright AD, Hill DM, Lowy C, Fraser TR. Mortality in acromegaly. Q J Med 1970;39:1-6.
Alexander L, Appleton D, Hall R, Ross WM, Wilkinson R. Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol (Oxf) 1980;12:71-79.
Richie CM, Atkinson AB, Kennedy AL et al. Ascertainment and natural history of treated acromegaly in Northern Ireland. Ulster Med Journal 1990;59:55-62.
Bengtsson B, Eden S, Ernest I, Oden A, Sjorgen B. Epidemiology and long term survival in acromegaly. Acta Med Scand 1988;223:327-335.
Extabe J, Gaztambide S, Latorre P, Vazquez JA. Acromegaly: An epidemiological study. J Endocrinol Invest 1993;16:181-187.
Rajasoorja C, Holdaway IM, Wrightson P, Scott DJ, Iddertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 1994;41:95-102.
Ezzat S, Melmed S. Clinical review 18: are patients with acromegaly at increased risk for neoplasia? J Clin Endocrinol Metab 1991;72:245-249.
Tindall G, Oyesiku N, Watts N, Clark R, Christy J, Adams P. Transsphenoidal adenomectomy for growth hormone-secreting pituitary adenomas in acromegaly: outcome analysis and determinants of failure. J Neurosurg 1993;78:205-215.
Liuzzi A, Dallabonanza D, Oppizzi PG. The medical treatment of acromegaly with octreotide. Basel Karger, 1996:74-89.
Bates AS, Van't Hoff W, Jones JM, Clayton RN. An audit of outcome of treatment in acromegaly. Q J Med 1993;86:293-299.
Melmed S, Herman-Dowling R, Frohman LA et al. Consensus statement: benefits versus risks of medical therapy for acromegaly. Am J Med 1994;97:468-473.
Lancranjan I, Bruns C, Grass P et al. Pharmacokinetics, pharmacodynamics, efficacy and tolerability in acromegalic patients. Metab Clin Exp 1995;44:18-26.
Grass P, Marbach P, Bruns C, Lancranjan I. Sandostatin(r) LAR(r) (microencapsulated octreotide acetate) in acromegaly: Pharmacokinetic and pharmacodynamic relationships. Metabolism 1996;45:27-30.
Priou A, Levesque G, Simonetta C et al. Long acting sandostatine(Sandostatin LAR) in the treatment of acromegaly [French]. Ann Endocrinol 1995;56:213-218.
Stewart PM, Kane KF, Stewart SE et al. Depot long-acting somatostatin analog (Sandostatin LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 1995; 80:3267-3272.
Flogstad AK, Halse J, Haldorsen T et al. Sandostatin LAR in acromegalic patients: a dose-range study. J Clin Endocrinol Metab 1995;80:3601-3607.
Lancranjan I, Bruns C, Grass P et al. Sandostatin(r) LAR(r): A promising therapeutic tool in the management of acromegalic patients. Metabolism 1996;45:18-26.
Flogstad AK, Halse J, Bakke S et al. Sandostatin LAR in acromegalic patients: long-term treatment. J Clin Endocrinol Metab 1997;81:23-28.
Tauber JP, Poncet MF, Harris AG et al. The impact of continuous subcutaneous infusion of octreotide on gallstone formation in acromegalic patients. J Clin Endocrinol Metab 1995;80:3262-3266.
Christensen ES, Weeke J, Orskov H et al. Continuous subcutaneous pump infusion of somatostatin analogue SMS 201-995 versus subcutaneous injection schedule in acromegalic patients. Clin Endocrin 1987;27:297-306.
Tauber P, Babin T, Tauber MT et al. Long-term effects of continuous subcutaneous infusion of the somatostatin analog octreotide in the treatment of acromegaly. J Clin Endocrinol Metabol 1989;68:917-924.
James RA, White MC, Chatterjee S, Marciaj H, Kendall-Taylor PA. Comparison of octreotide delivered by continuos subcutaneous infusion with intermittent injection in the treatment of acromegaly. Eur J Clin Invest 1992;22:554-561.
Caron P, Morange-Ramos I, Cogne M, Jaquet P. Three year follow-up of acromegalic patients treated with intramuscular slow-release Lanreotide. J Clin Endocrinol Metab 1997;82:18-22.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lancranjan, I., Atkinson, A.B. & Sandostatin® LAR® Group# Results of a European Multicentre Study with Sandostatin® LAR® in Acromegalic Patients. Pituitary 1, 105–114 (1999). https://doi.org/10.1023/A:1009980404404
Issue Date:
DOI: https://doi.org/10.1023/A:1009980404404