Abstract
Objective: To determine the effects of the new somatostatin analogue, lanreotide, in its prolonged released form (PR), in patients with acromegaly.
Design: Prospective open multicenter non comparative study.
Setting: Thirty-three university-affiliated medical centers.
Patients: One hundred sixteen acromegalic patients with active disease, of whom 58 patients complied with the protocol and completed the 12-month period treatment.
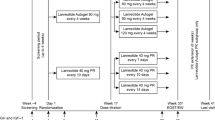
Intervention: Lanreotide PR treatment was started at a dose of 30 mg intramuscularly every 14 days. If integrated mean plasma GH levels were not below 5 μg/L and/or IGF-I levels were not normalized after one month of treatment, injections were given every 10 days. The duration of the study was 12 months.
Results: After one month of treatment mean plasma GH and IGF-I levels had fallen from 10.7 ± 11.1 μg/L (mean ± SD; range, 2.6 – 74.8 μg/L; median, 7 μg/L) and 718 ± 270 μg/L (range 338 – 1440 μg/L; median, 645 μg/L), respectively, to 7.8 ± 10.1 μg/L and 575 ± 252 μg/L, respectively. Thirty patients (22%) had plasma GH levels below 2.5 μg/L, and 8 patients (16%) had age-adjusted normal plasma IGF-I levels. At the sixth month of treatment mean plasma GH levels of 2.5 μg/L or less, and normal plasma IGF-I levels were observed in 33%, and 33% of patients, respectively. At the twelvth month of treatment, these percentages were 41%, and 41%, respectively. The interval between two injections was shortened (one injection every 10 days) in 8 of the 58 patients (13%) at the second month of treatment, and at the end of the study, 70% of patients required 3 injections per month. The most frequent adverse event elicited by enquiry was transient diarrhea (76% of patients), followed by abdominal pain (62%) and pain at the injection site (59%). Based on the analysis of a subgroup of 46 patients who had at least a measurement of fecal fat content after day 0 of the study, a non significant increase (from 4.2 ± 3.4 to 5.1 ± 4.3 g/24h, p = 0.3) in mean steatorrhea was observed during treatment. Before treatment, steatorrhea was present in 9 (19%) patients. During the study, 15 additional patients (32%) developed persistent steatorrhea, and there was a transient increase in fecal fat content above 6 g/24 h in another 11 patients. After exclusion of the 7 patients (12%) with gallstones at enrolment, new gallstones were diagnosed in 6 out of 50 patients (12%) during the study.
Conclusion: Two or three monthly injections of lanreotide PR decreased GH concentration to less than 2.5 μg/L and normalized IGF-I levels in 41% of patients treated during 12 months. The good tolerability of this treatment, and the reduction in the frequency of injections, plus the sustained drug serum concentrations, confirm the usefulness of this new somatostatin analog formulation.
Similar content being viewed by others
References
Melmed S. Acromegaly. In: Melmed S (ed). The Pituitary. Cambridge: Blackwell Science, 1995;413–442.
Melmed S, Ho K, Klibanski A, Reichlin S, Thorner M. Clinical review 75: Recent advances in pathogenesis, diagnosis, and management of acromegaly. J Clin Endocrinol Metab 1995;80:3395–3402.
Fahlbusch R, Honegger J, Buchfelder M. Surgical management of acromegaly. Endocrinol Metab Clin NorthAm 1992; 21:669–692.
Eastman RC, Gorden P, Glatstein E, Roth J. Radiation therapy of acromegaly. Endocrinol Metab Clin North Am 1992; 21:693–712.
Barkan AL, Halasz I, Dornfeld KJ, et al. Pituitary irradiation is ineffective in normalizing plasma insulin-like growth factor I in patients with acromegaly. J Clin Endocrinol Metab 1997;82:3187–3191.
Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med 1996;334:246–254.
Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumours. Clin Pharmacokinet 1993;25:375–391.
Gillis JC, Noble S, Goa KL. Octreotide long-acting release (LAR). A review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs 1997;53:681–699.
Sassolas G, Harris AG, James-Deidier A. Long term effect of incremental doses of the somatostatin analog SMS 201-995 in 58 acromegalic patients. French SMS 201–995 approximately equal to Acromegaly Study Group. J Clin Endocrinol Metab 1990;71:391–397.
Vance ML, Harris AG. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide. Results of the International Multicenter Acromegaly Study Group. Arch Intern Med 1991;151:1573–1578.
Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly. A randomized, multicenter study. Ann Intern Med 1992;117:711–718.
Newman CB, Melmed S, Snyder PJ, et al. Safety and ef~-cacy of long-term octreotide therapy of acromegaly: Results of a multicenter trial in 103 patients–a clinical research center study. J Clin Endocrinol Metab 1995;80:2768–2775.
Arosio M, Macchelli S, Rossi CM, Casati G, Biella O, Faglia G. Effects of treatment with octreotide in acromegalic patients–A multicenter Italian study. Italian Multicenter Octreotide Study Group. Eur J Endocrinol 1995;133:430–439.
Fl–gstad AK, Halse J, Bakke S, et al. Sandostatin LAR in acromegalic patients: Long-term treatment [see comments]. J Clin Endocrinol Metab 1997;82:23–28.
Stewart PM, Kane KF, Stewart SE, Lancranjan I, Sheppard MC. Depot long-acting somatostatin analog (Sandostatin-LAR) is an effective treatment for acromegaly. J Clin Endocrinol Metab 1995;80:3267–3272.
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR: A promising therapeutic tool in the management of acromegalic patients. Metabolism 1996;45:67–71.
Heron I, Thomas F, Dero M, et al. Pharmacokinetics and ef~cacy of a long-acting formulation of the new somatostatin analog BIM 23014 in patients with acromegaly. J Clin Endocrinol Metab 1993;76:721–727.
Morange I, De Boisvilliers F, Chanson P, et al. Slow release lanreotide treatment in acromegalic patients previously normalized by octreotide. J Clin Endocrinol Metab 1994;79:145–151.
Johnson MR, Chowdrey HS, Thomas F, Grint C, Lightman SL. Pharmacokinetics and ef~cacy of the long-acting somatostatin analogue somatuline in acromegaly. Eur J Endocrinol 1994;130:229–234.
Marek J, Hana V, Krsek M, Justova V, Catus F, Thomas F. Long-term treatment of acromegaly with the slow-release somatostatin analogue lanreotide. Eur J Endocrinol 1994;131:20–26.
Giusti M, Gussoni G, Cuttica CM, Giordano G. Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly: Six-month report on an Italian multicenter study. Italian Multicenter Slow Release Lanreotide Study Group. J Clin Endocrinol Metab 1996;81:2089–2097.
Frohman LA. Acromegaly: What constitutes optimal therapy–[editorial]. J Clin Endocrinol Metab 1996;81:443–445.
Consensus statement: Bene~ts versus risks of medical therapy for acromegaly. Acromegaly Therapy Consensus Development Panel. Am J Med 1994;97:468–473.
Gancel A, Vuillermet P, Legrand A, Catus F, Thomas F, Kuhn JM. Effects of a slow-release formulation of the new somatostatin analogue lanreotide in TSH-secreting pituitary adenomas. Clin Endocrinol (Oxf) 1994;40:421–428.
Caron P, Cogne M, Gusthiot-Joudet B, Wakim S, Catus F, Bayard F. Intramuscular injections of slow-release lan-reotide (BIM 23014) in acromegalic patients previously treated with continuous subcutaneous infusion of octreotide (SMS 201-995). Eur J Endocrinol 1995;132:320–325.
al-Maskari M, Gebbie J, Kendall-Taylor P. The effect of a new slow-release, long-acting somatostatin analogue, lanreotide, in acromegaly. Clin Endocrinol (Oxf) 1996;45:415–421.
Caron P, Morange-Ramos I, Cogne M, Jaquet P. Three year follow-up of acromegalic patients treated with intramuscular slow-release lanreotide. J Clin Endocrinol Metab 1997;82:18–22.
Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev 1995;16:427–442.
Hofland LJ, van Koetsveld PM, Waaijers M, Zuyderwijk J, Lamberts SW. Relative potencies of the somatostatin analogs octreotide, BIM-23014, and RC-160 on the inhibition of hormone release by cultured human endocrine tumor cells and normal rat anterior pituitary cells. Endocrinology 1994;134:301–306.
McKnight JA, McCance DR, Crothers JG, Atkinson AB. Changes in glucose tolerance and development of gall stones during high dose treatment with octreotide for acromegaly. Br Med J 1989;299:604–605.
Quabbe HJ, Plockinger U. Dose-response study and long term effect of the somatostatin analog octreotide in patients with therapy-resistant acromegaly. J Clin Endocrinol Metab 1989;68:873–881.
James RA, Moller N, Chatterjee S, White M, Kendall-Taylor P. Carbohydrate tolerance and serum lipids in acromegaly before and during treatment with high dose octreotide. Diabet Med 1991;8:517–523.
Koop BL, Harris A G, Ezzat S. Effect of octreotide on glucose tolerance in acromegaly. Eur J Endocrinol 1994;130:581–586.
Lamberts SW, Uitterlinden P, del Pozo E. SMS 201-995 induces a continuous decline in circulating growth hormone and somatomedin-C levels during therapy of acromegalic patients for over two years. J Clin Endocrinol Metab 1987;65:703–710.
McGregor AR, Troughton WD, Donald RA, Espiner EA. Effect of the somatostatin analogue SMS 201-995 on faecal fat excretion in acromegaly. Horm Metab Res 1990;22:55–56.
Ho PJ, Boyajy LD, Greenstein E, Barkan AL. Effect of chronic octreotide treatment on intestinal absorption in patients with acromegaly. Dig Dis Sci 1993;38:309–315.
Fredstorp L, Pernow Y, Werner S. The short and long-term effects of octreotide on calcium homeostasis in patients with acromegaly. Clin Endocrinol (Oxf) 1993;39:331–336.
Erlinger S, Chanson P, Dumont M, Ponsot P, Warnet A, Harris AG. Effects of octreotide on biliary lipid composition and occurrence of cholesterol crystal in patients with acromegaly. A prospective study. Dig Dis Sci 1994;39:2384–2388.
Catnach SM, Anderson JV, Fairclough PD, et al. Effect of octreotide on gall stone prevalence and gall bladder motility in acromegaly. Gut 1993;34:270–273.
Ho KY, Weissberger AJ, Marbach P, Lazarus L. Therapeutic ef~cacy of the somatostatin analog SMS 201-995 (octreotide) in acromegaly. Effects of dose and frequency and long-term safety. Ann Intern Med 1990;112:173–181.
Montini M, Gianola D, Pagani MD, et al. Cholelithiasis and acromegaly: Therapeutic strategies. Clin Endocrinol (Oxf) 1994;40:401–406.
McKnight JA, McCance DR, Sheridan B, et al. A long-term dose-response study of somatostatin analogue (SMS 201-995, octreotide) in resistant acromegaly. Clin Endocrinol (Oxf) 1991;34:119–125.
Dowling RH, Hussaini SH, Murphy GM, Besser GM, Wass JA. Gallstones during octreotide therapy. Metabolism 1992;41:22–33.
Shi YF, Zhu XF, Harris AG, Zhang JX, Dai Q. Prospective study of the long-term effects of somatostatin analog (octreotide) on gallbladder function and gallstone formation in Chinese acromegalic patients. J Clin Endocrinol Metab 1993;76:32–37.
Tauber JP, Babin T, Tauber MT, et al. Long term effects of continuous subcutaneous infusion of the somatostatin analog octreotide in the treatment of acromegaly. J Clin Endocrinol Metab 1989;68:917–924.
Page MD, Millward ME, Taylor A, et al. Long-term treatment of acromegaly with a long-acting analogue of somatostatin, octreotide. Q JMed 1990;74:189–201.
Eastman RC, Arakaki RF, Shawker T, et al. A prospective examination of octreotide-induced gall-bladder changes in acromegaly. Clin Endocrinol (Oxf) 1992;36:265–269.
Tauber JP, Poncet MF, Harris AG, et al. The impact of continuous subcutaneous infusion of octreotide on gallstone formation in acromegalic patients. J Clin Endocrinol Metab 1995;80:3262–3266.
Plockinger U, Dienemann D, Quabbe HJ. Gastrointestinal side-effects of octreotide during long-term treatment of acromegaly. J Clin Endocrinol Metab 1990;71:1658–1662.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chanson, P., Leselbaum, A., Blumberg, J. et al. Efficacy and Tolerability of the Long-Acting Somatostatin Analog Lanreotide in Acromegaly. A 12-Month Multicenter Study of 58 Acromegalic Patients. Pituitary 2, 269–276 (2000). https://doi.org/10.1023/A:1009961116472
Issue Date:
DOI: https://doi.org/10.1023/A:1009961116472