Abstract
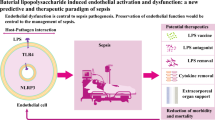
In recent years research on peritonitis has focused on the mesothelial lining of the peritoneal cavity. Activation of this cell-layer by direct contact with micro-organisms is known to influence coagulation, fibrinolysis and inflammatory processes. This review summarizes the recent advances in activation mechanisms of mesothelial cells by micro-organisms. Between bacterial species striking differences in activation of mesothelium exist, resulting in a variey of effects. In future these insights may lead to new therapeutic options for bacterial peritonitis.
Similar content being viewed by others
References
Bouchet Y, Voilin C, Yver R. The peritoneum and its anatomy. In: Bengmark S, ed. The Peritoneum and Peritoneal Access. London: Wright, 1989:1–13.
Dobbie JW. New concepts in molecular biology and ultrastructural pathology of the peritoneum: Their signi~cance for peritoneal dialysis. Am J Kidney Dis 1990;15:97–109.
Zeillemaker Am, Mul FPJ, Hoynck van Papendrecht AAGM, Leguit P, Verbrugh HA, Roos D. Neutrophil adherence to and migration across monolayers of human peritoneal mesothelial cells. J Lab Clin Med 1996;127:279–286.
Hinsbergh van VWM, Kooistra T, Scheffer MA, Bockel van JH, Muijen van GNP. Characterization and ~brinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood 1990;75:1490–1497.
Nespoli A, Ravizzini C, Trivella L, Segala M. The choice of surgical procedure for peritonitis due to colonic perforation. Arch Surg 1993;128:814–818.
McDougal WS, Izant RJ Jr, Zollinger RM Jr, Primary peritonitis in infancy and childhood. Ann Surg 1975;181:310–313.
Wyke RJ. Problems of bacterial infection in patients with liver disease. Gut 1987;28:623–641.
Correia JP, Conn HO. Spontaneous bacterial peritonitis in cirrhosis: endemic or epidemic? Med Clin North Am 1975; 59:963–981.
Mal F, Pham Huu T, Bendahou M, Trinchet JC, Garnier M, Hakim J, Beaigrand M. Chemoattractant and opsonic activity in ascitic _uid. J Hepathology 1991;12:45–49.
Rotstein OD, Timothy LP, Simmons RL. Lethal microbial synergism in intra-abdominal infections. Arch Surg 1985; 120:146–151.
Peach SL, Tabaqchali S. Mucosa-associated _ora of the human gastrointestinal tract in health and disease. Eur J Chemoth Antibiot 1982;3:41–50.
Lorber B, Swenson RM. The bacteriology of intra-abdominal infections. Surg Clin North Am 1975;55:1349–1354.
Verbrugh, HA. Organisms caising peritonitis. Contrib Nephrol 1990;85:39–48.
Rotstein OD, Pruett TL, Simmons RL. Microbiologic features and treatment of persistent peritonitis in patients in the intensive care unit. Can J Surg 1986;29:247–250.
Haagen IA, Heezius HCJM, Verkooyen RP, Verhoeff J, Verbrugh HA. Adherence of Staphylococci caising peritonitis to human peritoneal mesothelial cell monolayers. J Infect Dis 1990;161:226–273.
Muijsken MA, Heezius HJ, Verhoef J, Verbrugh HA. Role of mesothelial cells in peritoneal antibacterial defence. J Clin Pathol 1991;44:600–604.
Glancey G, Cameron JS, Ogg C, Poston S. Adherence of Staphylococcus aireus to cultures of human peritoneal mesothelial cells. Nephrol Dial Transplant 1993;8:157–162.
Muijsken MA, de Fijter CHW, Heezius ECJ, Oe PL, Verhoef J, Verbrugh HA. Intracellular survival of Staphylococci in both peritoneal macrophages and mesothelial cells as a possible caise of relapsing peritonitis. J Am Soc Nephrol 1991;2:336–339
Edminson CE, Goheen MP, Kornhall S, Jones FE, Condon RE. Fecal peritonitis: microbial adherence to serosal mesothelium and resistance to peritoneal lavage. World J Surg 1990;14:176–183.
Jonjic N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Montovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med 1992;176:1165–1174.
Lots M, Jirik F, Kabouridis R, Tsoukas C, Hirano T, Kishimoto T, Carson DA. BSF-2/IL-6 is costimulant for human thymocytes and T-lymphocytes. J Exp Med 1988;140: 508–513.
Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, et al. Complementary DAN for novel human interleukin (BSF-2) that induces lymphocytes to produce immunoglobin. Nature 1986;324:73–77.
Luger TA, Krutmann J, Kirnbaner R, Vrbarski A, Schwartz T, Klappacher G, Kock A, et al. IFN-b2/IL-6 aigments the activity of human natural killer cells. J Immunol 1989; 143:1206–1210.
Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 1989;84:1045–1049.
Zeillemaker AM, Mul FPJ, Hoynck van Papendrecht AAGM, Kuijpers TW, Roos D, Leguit P, Verbrugh HA. Polarized secretion of IL-8 by human mesothelial cells: A role in neutrophil migration. Immunology 1995;84: 227–232.
Boylan AM, Rüegg C, Kim KJ, Hébert CA, Hoeffel JM, Pytela R, Sheppard D, Goldstein IM, Broaddus CV. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenisis of asbestos-induced pleurisy in rabbits. J Clin Invest 1992; 89:1257–1262.
Lin CY, Lin CC, Huang TP. Serial changes of Interleukin-6 and interleukin-8 levels in drain dialysate of uremic patients with continuous ambulatory peritoneal dialysis during peritonitis. Nephron 1993;63: 404–408.
Visser CE, Steenbergen JJE, Betjes MCH, Meijer S, Arisz L, Hoefsmit ECM, Krediet RT, Beelen RHJ. Interleukin-8 production by human mesothelial cells after direct stimulation with Staphylococci. Infect Immun 1995;63: 206–4209.
Kinnaert P, de Wilde JP, Bournonville B, Husson C, Salmon I. Direct activation of human peritoneal mesothelial cells by heat-killed microorganisms. Ann Surg 1996;6:749–755.
Zeillemaker AM, Hoynck van Papendrecht AAGM, Hart MHL, Roos D, Verbrugh HA, Leguit P. Peritoneal interleukin-8 in acute peritonitis. J Surg Res 1996;62:273–277.
Westreenen M, Pronk A, Diepersloot RJA, de Groot, Ph. G, Leguit P. A Chlamydia trachomatis infection of human mesothelial cells alters pro-in_ammatory, procoagulant, and ~brinolytic responses. Infect Immun 1988;66:2352–2355.
Jönsson B, Berglund J, Skai T, Nyström. Outcome of intraabdominal infection in pigs depends more on host responses than on microbiology. Eur J Surg 1993;159:571–578.
Schoeffel U, Jacobs E, Ruf G, Mierswa F, von Specht BU, Farthmann. Intraperitoneal micro-organisms and the severity of peritonitis. Eur J Surg 1995;161: 501–508.
Poxton IR, Edmond DM. Biological activity of bacteroides lipopolysaccharide-reappraisal. Clin Infect Dis 1995;20 (suppl 2):s149–153.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeillemaker, A.M., Diepersloot, R.J. & Leguit, P. Mesothelial Cell Activation by Micro-Organisms. Sepsis 3, 285–291 (1999). https://doi.org/10.1023/A:1009897209650
Issue Date:
DOI: https://doi.org/10.1023/A:1009897209650