Abstract
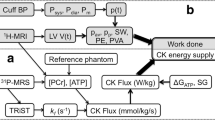
This chapter examines the role of cardiac high-energy phosphate metabolism in normal, hypertrophied and failing human myocardium. Myocardial biopsies allow analysis of ATP, total adenine nucleotides, creatine kinase activity and total creatine content, while non-invasive 31P-magnetic resonance spectroscopy can be used to determine phosphocreatine/ATP ratios and, most recently, absolute levels of ATP and phosphocreatine. The failing human myocardium is characterized by reduced phosphocreatine and total creatine levels, normal or slightly reduced ATP levels and reduced creatine kinase activity. These changes are consistent with, but do not prove, a role of high-energy phosphate metabolism as a contributing factor in heart failure. An answer to the precise functional role of high-energy phosphate metabolism necessitates analysis of free ADP levels, free energy change of ATP hydrolysis and creatine kinase reaction velocity; these measurements may become feasible in coming years. However, analysis of energy metabolism in a compartmentalized manner, i.e., in the compartments relevant for contractile function such as the perimyofibrillar space, will remain elusive for the foreseeable future.
Similar content being viewed by others
References
Ingwall JS. Is cardiac failure a consequence of decreased energy reserve? Circulation 1993;87(Suppl VII):58–62.
Clarke K, O'Connor AJ, Willis RJ. Temporal relation between energy metabolism and myocardial function during ischemia and reperfusion. Am J Physiol 1987;253:H412–421.
Swain JL, Sabina RL, Peyton RB, Jones RN, Wechsler AS, Holmes EW. Derangements in myocardial purine and pyrimidine nucleotide metabolism in patients with coronary artery disease and left ventricular hypertrophy. Proc Natl Acad Sci USA 1982;79:655–659.
Peyton RB, Jones RN, Attarian D, Sink JD, van Trigt P, Currie WD, Wechsler AS. Depressed high-energy phosphate content in hypertrophied ventricles of animal and man. Ann Surg 1982;196:278–284.
Starling RC, Hammer DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem 1998;180:171–177.
Bashore TM, Magorien DJ, Letterio J, Shaffer P, Unverferth DV. Histologic and biochemical correlates of left ventricular chamber dynamics in man. J Am Coll Cardiol 1987;9: 734–742.
Regitz V, Fleck E. Myocardial adenine nucleotide concentrations and myocardial norepinephrine content in patients with heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 1992;69:1574–1580.
Neubauer S, Ingwall JS. Verapamil attenuates ATP depletion during hypoxia: 31P NMR studies of the isolated rat heart. J Mol Cell Cardiol 1989;21:1163–1178.
Kammermeier H. Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Anal Biochem 1973;56:341–345.
Horn M, Frantz S, Remkes H, Laser A, Urban B, Metten-Neubauer leiter A, Schnackerz K, Neubauer S. Effects of chronic dietary creatine feeding on cardiac energy metabolism and on creatine content in heart, skeletal muscle, brain, liver and kidney. J Mol Cell Cardiol 1998;30:277–284.
Neubauer S, Hamman BL, Perry SB, Bittl JA, Ingwall JS. Velocity of the creatine kinase reaction decreases in postischemic myocardium: A 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res 1988;63:1–15.
Neubauer S, Frank M, Hu K, Remkes H, Laser A, Horn M, Ertl G, Lohse MJ. Changes of creatine kinase gene expression in residual intact myocardium of chronically infarcted rats. J Mol Cell Cardiol 1998;30:803–810.
Hoang CD, Zhang J, Payne RM, Apple FS. Post-infarction left ventricular remodeling induces changes in creatine kinase mRNA and protein subunit levels in porcine myocardium. Am J Pathol 1997;151(1):257–264.
Lowry OH, Rosebrough MJ, Farr AL, Randall RJ. Protein measurement with the foline reagent. J Biol Chem 1951; 193:265.
Bottomley PA. MR spectroscopy of the human heart: the status and the challenges. Radiology 1994;191:593–612.
von Kienlin M, Mejia R. Spectral localization with optimal pointspread function. Magn Reson Med 1991;94:268–287.
Meininger M, Landschütz W, Beer M, Seyfarth T, Horn M, Pabst T, Haase A, Hahn D, Neubauer S, von Kienlin M. Concentrations of human cardiac phosphorus metabolites determined by SLOOP 31P NMR spectroscopy. MRM 1999;41:657–663.
Hetherington HP, Luney DJ, Vaughan JT, Pan JW, Ponder SL, Tschendel O, Twieg DB, Pohost GM. 3D 31P spectroscopic imaging of the human heart at 4.1 T. Magn Reson Med 1995;33:427–431.
Menon RS, Hendrich K. 31P NMR spectroscopy of human heart at 4T: Detection of substantially uncontaminated cardiac spectra and differentiation of subepicardium and subendocardium. Magn Reson Med 1992;26:368–376.
Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. The creatine kinase system in failing and nonfailing human myocardium. Circulation 1996;94:1894–1901.
Auffermann W, Chew WM, Wolfe CL, Tavares NJ, Parmley WW, Semelka RC, Donnelly T, Chatterjee K, Higgins CB. Normal and diffusely abnormal myocardium in humans: Functional and metabolic characterization with P-31 MR spectroscopy and cine MR imaging. Radiology 1991;179: 253–259.
Bottomley PA, Hardy CJ, Roemer PB. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med 1990;14:425–434.
Bottomley PA, Atalar E, Weiss RG. Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med 1996;35:664–670.
Neubauer S, Krahe T, Schindler R, Hillenbrand H, Entzeroth C, Horn M, Bauer WR, Stephan T, Lackner K, Haase A, et al. Direct measurement of spin-lattice relaxation times of phosphorus metabolites in human myocardium [see comments]. Magn Reson Med 1992;26:300–307.
van Dobbenburgh JO, Lekkerkerk C, van Echteld CJ, de Beer R. Saturation correction in human cardiac 31P MR spectroscopy at 1.5 T. NMR Biomed 1994;7:218–224.
Hardy CJ, Bottomley PA, Rohling KW, Roemer PB. An NMR phased array for human cardiac 31P spectroscopy. Magn Reson Med 1992;28:54–64.
Bottomley PA, Hardy CJ. Mapping creatine kinase reaction rates in human brain and heart with 4 tesla saturation transfer 31P NMR. J Magn Reson 1992;99:443–448.
Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med 1985;313:1050–1054.
Bottomley PA, Weiss RG. Non-invasivemagnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet 1998;351:714–718.
Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986;232:1121–1123.
Lamb HJ, Beyerbacht HP, R. O, Doornbos J, B.M. P, van der Wall EE, van der Laarse A, de Roos A. Metabolic response of normal human myocardium to high-dose atropine-dobutamine stress studied by 31P-MRS. Circulation 1997;96: 2969–2977.
Okada M, K. M, Inubushi T, Kinoshita M. Influence of aging or left ventricular hypertrophy on the human heart: Contents of phosphorus metabolites measured by 31P MRS. MRM 1998;39:772–782.
Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van derWall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 1999;99:2261–2267.
Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine toATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 1991;338:973–976.
Neubauer S, Horn M, Pabst T, Harre K, Strömer H, Bertsch G, Sandstede J, Ertl G, Hahn D, Kochsiek K. Cardiac highenergy phosphate metabolism in patients with aortic valve disease assessed by 31P-magnetic resonance spectroscopy. J Invest Med 1997;45:453–462.
Conway MA, Bottomley PA, Ouwerkerk R, Radda GK, Rajagopalan B. Mitral regurgitation: Impaired systolic function, eccentric hypertrophy, and increased severity are linked to lower phosphocreatine/ATP ratios in humans. Circulation 1998;97:1716–1723.
Zhang J, Merkle H, Hendrich K, Garwood M, From AH, Ugurbil K, Bache RJ. Bioenergetic abnormalities associated with severe left ventricular hypertrophy. J Clin Invest 1993;92:993–1003.
Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. New Engl J Med 1997;336/11:775–785.
Spindler M, Saupe KW, Christe ME, Seidman CE, Seidman JG, Ingwall JS. A murine model of familial hypertrophic cardiomyopathy shows a markedly impaired response to inotropic stimulation. Circulation 1996;94/8(Suppl I):I–433 (Abstract).
Rajagopalan B, Blackledge MJ, McKenna WJ, Bolas N, Radda GK. Measurement of phosphocreatine to ATP ratio in normal and diseased human heart by 31P magnetic resonance spectroscopy using the rotating frame-depth selection technique. Ann NY Acad Sci 1987;508:321–332.
Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease [see comments]. N Engl J Med 1990;323:1593–1600.
Masuda Y, Tateno Y, Ikehira H, Hashimoto T, Shishido F, Sekiya M, Imazeki Y, Imai H, Watanabe S, Inagaki Y. Highenergy phosphate metabolism of the myocardium in normal Energy Metabolism in Human Heart Failure subjects and patients with various cardiomyopathies—the study using ECG gated MR spectroscopy with a localization technique. Jpn Circ J 1992;56:620–626.
de Roos A, Doornbos J, Luyten PR, Oosterwaal LJ, van der Wall EE, den Hollander JA. Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: Assessment with proton-decoupled P-31 MR spectroscopy. J Magn Reson Imaging 1992;2:711–719.
Sakuma H, Takeda K, Tagami T, Nakagawa T, Okamoto S, Konishi T, Nakano T. 31P MR spectroscopy in hypertrophic cardiomyopathy: Comparison with T1-201myocardial perfusion imaging. Am Heart J 1993;125:1323–1328.
Sieverding L, Jung WI, Breuer J, Widmaier S, Staubert A, van Erckelens F, Schmidt O, Bunse M, Hoess T, Lutz O, Dietze GJ, Apitz J. Proton-decoupled myocardial 31P NMR spectroscopy reveals decreased PCr/Pi in patients with severe hypertrophic cardiomyopathy. Am J Cardiol 1997;80(3A):34A–40A.
Jung WI, Sieverding L, Breuer J, Hoess T, Widmaier S, Schmidt O, Bunse M, van Erckelens F, Apitz J, Lutz O, Dietze GJ. 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 1998;97:2536–2542.
Pluim BM, Chin JC, De Roos A, Doornbos J, Siebelink HM, Van der Laarse A, Vliegen HW, Lamerichs RM, Bruschke AV, Van der Wall EE. Cardiac anatomy, function and metabolism in elite cyclists assessed by magnetic resonance imaging and spectroscopy [see comments]. Eur Heart J 1996;17:1271–1278.
Pluim BM, Lamb HJ, Kayser HW, Leujes F, Beyerbacht HP, Zwinderman AH, van der Laarse A, Vliegen HW, de Roos A, van der Wall EE. Functional and metabolic evaluation of the athlete's heart by magnetic resonance imaging and dobutamine stress magnetic resonance spectroscopy. Circulation 1998;97:666–672.
Spencer RG, Buttrick PM, Ingwall JS. Function and bioenergetics in isolated perfused trained rat hearts. Am J Physiol 1997;272:H409–417.
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 1991;122:795–801.
Schaefer S, Gober JR, Schwartz GG, Twieg DB, Weiner MW, Massie B. In vivo phosphorus-31 spectroscopic imaging in patients with global myocardial disease. Am J Cardiol 1990;65:1154–1161.
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphatemetabolism in heart failure. Circulation 1992;86:1810–1818.
Neubauer S, Horn M, Pabst T, Gödde M, Lübke D, Illing B, Hahn D, Ertl G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 1995;16 (Suppl O):115–118.
Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. In patients with dilated cardiomyopathy the myocardial phosphocreatine-to-ATP ratio is a predictor of mortality. Circulation 1997;96:2190–2196.
Meininger M, Landschütz W, Beer M, Seyfarth T, Bertsch G, Sandstede J, Pabst T, Horn M, Haase A, Hahn D, von Kienlin M, Neubauer S. Nichtinvasive Messung der Absolutkonzentrationen der energiereichen Phosphate am gesunden und insuf~zienten menschlichen Myokard mit 31P-SLOOPMRS. Z Kardiol 1999;88:278 (Abstract).
Cohn J, Bristow MR, Chien KR, Colucci WS, Frazier H, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Leinrib L. Report of the national heart, lung, and blood institute special emphasis panel on heart failure research. Circulation 1997;95:766–770.
Blackledge MJ, Rajagopalan B, Oberhaensli RD, Bolas NM, Styles P, Radda GK. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci USA 1987;84:4283–4287.
Schaefer S, Gober J, Valenza M, Karczmar GS, Matson GB, Camacho SA, Botvinick EH, Massie B, Weiner MW. Nuclear magnetic resonance imaging-guided phosphorus-31 spectroscopy of the human heart. J Am Coll Cardiol 1988;12:1449–1455.
Yabe T, Mitsunami K, Okada M, Morikawa S, Inubushi T, Kinoshita M. Detection of myocardial ischemia by 31P magnetic resonance spectroscopy during handgrip exercise. Circulation 1994;89:1709–1716.
Yabe T, Mitsunami K, Inubushi T, Kinoshita M. Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy [see comments]. Circulation 1995;92:15–23.
Kalil-Filho R, de Albuquerque CP, Weiss RG, Mocelim A, Bellotti G, Cerri G, Pileggi F. Normal high-energy phosphate ratios in stunned human myocardium. J Am Coll Cardiol 1997;30:1228–1232.
Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96:2190–2196.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neubauer, S. High-Energy Phosphate Metabolism in Normal, Hypertrophied and Failing Human Myocardium. Heart Fail Rev 4, 269–280 (1999). https://doi.org/10.1023/A:1009866108384
Issue Date:
DOI: https://doi.org/10.1023/A:1009866108384