Abstract
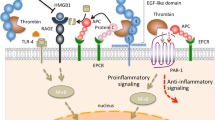
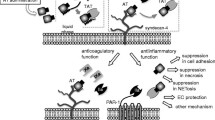
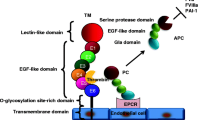
Tissue Factor Pathway Inhibitor (TFPI) is an endogenous inhibitor of the extrinsic pathway of coagulation. TFPI is a serine protease inhibitor that inhibits factor Xa directly and the factor VIIa/tissue factor catalytic complex in a Xa dependent fashion. TFPI is a multivalent inhibitor with three tandemly arranged Kunitz-type protease inhibitor domains, that appears to provide the protein with distinct functional properties, which make the inhibitor well suited for a regulatory role. TFPI is synthesized and secreted by endothelial cells. Circulating TFPI is lipoprotein associated. Control of thrombus formation in the microvasculature is likely to lead to the development of therapeutic modalities for the treatment of severe sepsis, especially when the thrombotic process is known to be a stimulus for multiple organ failure. A large pool of endogenous TFPI is found bound to the endothelium, while a small pool is stored in platelets.
The concept of utilizing TFPI to protect the integrity of microvasculature, inhibit the initiation and propagation of multiple organ failure through the reduction of microthrombi formation, was examined in several models of sepsis, with and without severe DIC. There was significant survival benefit by the administration of recombinant TFPI to septic animals, in bacteremia and peritonitis models, several hours after the onset of symptoms. TFPI was found to exhibit anti-inflammatory activities as demonstrated by the decrease of IL-6 and IL-8 levels in the treated animals. TFPI was reported to bind endotoxin in vitro and reduce Fas levels in vivo suggesting the attenuation of apoptosis, in the target microvascular endothelial cells.
TFPI was tested for safety and tolerability in normal human volunteers and more recently in patients with severe sepsis. The human clinical data suggest that TFPI is safe and well tolerated. TFPI is currently in phase II studies in severe sepsis patients.
Similar content being viewed by others
References
van Deventer SJH, Buller HR, ten Cate JW, et al. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic and complement pathways. Blood 1990;76:2520–2526.
van der Poll T. Cytokines and their inhibition in septicemias. Br J Intensive Care 1992;2:99–110.
van Deuren M, van der Ven-Jonge-Krijg J, De macker PNM, et al. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Disease 1994;169:157–161.
Conway EM, Bach R, Rosenberg RD, Konigsberg WH. Tumor necrosis factor enhances expression of tissue factor mRNAin endothelial cells. Thrombosis Res 1989;53:231–241.
Drake TA, Ruf W, Morrissey JH, Edgington TS. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide stimulated human blood monocytes and a constitutively tissue factor producing neoplastic cell line. J Cell Biol 1989;109:389–395.
Nemerson Y. Tissue factor: Then and now. Thromb Haemost 1995;71:1–8.
Buzan J. Structural design and molecular evaluation of a cytokine receptor family. J Proc Natl Acad Sci USA 1990;87:6934–6938.
Shimura M, Wada H, Wakita Y, et al. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol 1997;55:169–174.
Seligsohn D, Kasper CK, Osterud B, Rapaport SI. Activated factor VII: Presence in factor IX concentrates and persistence in circulation after infusion. Blood 1978;58:828–837.
Petersen LC, Valentin S, Hedner U. Regulation of the extrinsic pathway system in health and disease: The role of factor VIIa and tissue factor pathway inhibitor. Thromb Res 1995;79:1–47.
Hjort PF. Intermediate reactions in the coagulation of blood with tissue thromboplastin. Scand J Clin Lab Invest 1957;9(27):1–182.
Wun TC, Kretzmer KK, Girard TJ, et al. Cloning and characterization of a cDNA coding for the lipoprotein associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J Biol Chem 1988;263:6001–6004.
Novotny WF, Girard TJ, Miletich JP, Broze GJ. Purification and characterization of lipoprotein associated coagulation inhibitor from human plasma. J Biol Chem 1989;264:18832–18837.
Enjyouji KI, Emi E, Mukai T, et al. Human tissue factor pathway inhibitor (TFPI) gene: Complete genomic structure and localization on the genomic map of chromosome 2q. Genomics 1993;17:423–428.
Broze GJ, Miletich JP. Characterization of the inhibition of tissue factor in serum. Blood 1987;69:150–155.
Sanders NL, Bajaj SP, Zivelin A, Rapaport SI. Inhibition of tissue factor/factor VIIa activity in plasma requires factor X and an additional plasma component. Blood 1985;66:204–212.
Broze GJ Jr. Tissue factor pathway inhibitor and the revised theory of coagulation. Ann Rev Med 1995;46:103–112.
Girard TJ, Warren LA, Novotny WF, et al. Functional significance of the Kunitz type inhibitor domains of lipoprotein associated coagulation inhibitor. Nature 1989;338:518–520.
Valentin S, Nordfang O, Bregengard C, Wildgoose P. Evidence that the C-terminus of tissue factor pathway inhibitor (TFPI) is essential for its in vitro and in vivo interaction with lipoproteins. BloodCoagul Fibrinolysis 1993;4:713–720.
Enjyoji K, Miyata T, Kamikubo Y, Kato H. Effect of heparin on the inhibition of factor Xa by tissue factor pathway inhibitor: Asegment, Gly212–Phe243, of the third Kunitz domain is a heparin-binding site. Biochemistry 1995;34:5725–5735.
Wesselschmidt R, Likert K, Huang Z, et al. Structural requirements for tissue factor pathway inhibitor interactions with factor Xa and heparin. Blood Coagul Fibrinolysis 1993;4:661–669.
Iversen N, Sandset PM, Abildgaard U, Torjesen PA. Binding of tissue factor pathway inhibitor to cultured endothelial cells. Influence of glycosaminoglycans. Thromb Res 1996;84:267–278.
Huang Z-F, Wun T-C, Broze GJ Jr. Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem 1993;268:26950–26955.
BajajMS, Kuppuswamy MN, Saito H, et al. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: Evidence that the endothelium is the principal site of synthesis. J Proc Natl Acad Sci USA 1990;87:8869–8873.
Ameri A, Kuppuswamy MN, Basu S, Bajaj SP. Expression of tissue factor pathway inhibitor by cultured endothelial cells in response to inflammatory mediators. Blood 1992;79:3219–3226.
Werling RW, Zacharski LR, Kisiel W, et al. Distribution of tissue factor pathway inhibitor in normal and malignant human tissues. Thromb Haemost 1993;69:366–369.
Lindahl AK, Jacobsen PB, Sandset PM, Abildgaard U. Tissue factor pathway inhibitor with high anti-coagulant activity is increased in post-heparin plasma and in plasma from cancer patients. Blood Coag Fibrinol 1991;2:713–722.
Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI). Thromb Res 1988;50:803–813.
Novotny WF, Girard TJ, Miletich JP, Broze GJ. Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein-associated coagulation inhibitor. Blood 1988;71:2020–2025.
Lindahl AK, Jacobsen PB, Sandset PM, Abildgaard U. Tissue factor pathway inhibitor with high anticoagulant activity is expressed in post-heparin plasma and in plasma from cancer patients. Blood Coag Fibrinolysis 1991;2:713–721.
Llobet D, Falkon L, Mateo J, et al. Low levels of tissue factor pathway inhibitor (TFPI) in two out of three members of a family with thrombophilia. Thromb Res 1995;80:413–418.
Day KC, Hoffman LC, Palmier MO, et al. Recombinant lipoprotein-associated coagulation inhibitor inhibits tissue thromboplastin-induced intravascular coagulation in the rabbit. Blood 1990;76:1538–1545.
Palmier MO, Hall LJ, Reisch CM, et al. Clearance of recombinant tissue factor pathway inhibitor (TFPI) in rabbits. Thromb Haemost 1992;68:33–36.
Bregengaard C, Nordfang O, Ostergaard P, et al. Pharmacokinetics of full length and two-domain tissue factor pathway inhibitor in combination with heparin in rabbits. Thromb Haemost 1993;70:454–457.
Warshawsky I, Bu G, Mast A, et al. The carboxyterminus of tissue factor pathway inhibitor is required for interacting with hepatoma cells in vitro and in vivo. J Clin Invest 1995;95:1773–1781.
Narita M, Bu G, Olins GM, et al. Two receptor systems are involved in the plasma clearance of tissue factor pathway inhibitor in vivo. J Biol Chem 1995;270:24800–24804.
Ho G, Toomey JR, Broze GJ Jr, Schwartz AL. Receptor mediated endocytosis of coagulation factor Xa requires cell surface-bound tissue factor pathway inhibitor. J Biol Chem 1996;271:9497–9502.
Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI). Thromb Res 1988;50:803–813.
Lindahl AK, Abildgaard U, Staalesen R. The anticoagulant effect in heparinized blood and plasma resulting from interactions with extrinsic pathway inhibitor. Thromb Res 1991;64:155–168.
Novotny WF, Brown SG, Miletich JP, et al. Plasma antigen levels of the lipoprotein associated coagulation inhibitor in patient samples. Blood 1991;78:387–393.
Holst J, Lindblad B, Bergquist D, et al. Effect of protamine sulfate on tissue factor pathway inhibitor released by iv or sc standard or low molecular weight heparin. Thromb Haemost 1993;69:1114.
Mast AE, Highuchi DA, Huang ZF, et al. Glypican-3 is a binding protein on the HepG2 cell surface for tissue factor pathway inhibitor. Biochem J 1997;327:557–583.
Iversen N, Sandset PM, Abildgaard U, Torjesen PA. Binding of tissue factor pathway inhibitor to cultured endothelial cells. Influence of glycosaminoglycans. Thromb Res 1996;84:267–278.
Wun T-C, Kretzmer KK, Palmier MO, et al. Comparison of recombinant lipoprotein-associated coagulation inhibitors expressed in human SK hepatoma, mouse C127, baby hamster kidney, and Chinese hamster ovary cells. Thromb Haemost 1992;68:54–59.
Higuchi DA, Wun T-C, Likert KM, Broze GJ Jr. The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood 1992;79:1712–1719.
Petersen LC, Bjorn SE, Nordfang O. Effect of leukocyte proteinases on tissue factor pathway inhibitor. Thromb Haemost 1992;67:537–541.
Ohkura N, Enjyoji K, Kamikubo Y, Kato H. A novel degradation pathway of tissue factor pathway inhibitor: Incorporation into fibrin clot and degradation by thrombin. Blood 1997;90:1883–1892.
Li A, Wun T-C. Proteolysis of tissue factor pathway inhibitor (TFPI) by plasmin: Effect of TFPI activity. Thromb Haemost 1998;80:423–427.
Salemink I, Franssen J, Willems GM, et al. Factor Xa cleavage of tissue factor pathway inhibitor is associated with loss of anticoagulant activity. Thromb Haemost 1998;80:273–280.
Broze GJ Jr, Lange GW, Duffin KL, MacPhail L. Heterogeneity of plasma tissue factor pathway inhibitor. Blood Coag Fibrinolysis 1994;5:551–559.
Sevinsky JR, Rao LVM, Ruf W. Ligand-induced protease receptor translocation into caveolae: A mechanism for regulating cell surface proteolysis of the tissue factor dependent coagulation pathway. J Cell Biol 1996;133:293–304.
Sandset PM, Larsen ML, Abildgaard U, Lindahl AK, Odegaard OR. Chromogenic substrate assay of extrinsic pathway inhibitor (EPI): Levels in the normal human population and relation to cholesterol. Blood Coag Fibrinolysis 1991;2:425–433.
Hansen JB, Huseby KR, Huseby NE, Ezban M, Nordoy A. Tissue factor pathway inhibitor in complex with low density lipoprotein isolated from human plasma does not possess anticoagulant function in tissue factor-induced coagulation in vitro. Thromb Res 1997;85:413–425.
Wun T-C, Hyang MD, Kretzmer KK, et al. Immunoaffinity puri~cation and characterization of lipoprotein associated coagulation inhibitor from HepG2 hepatoma, chang liver, and SK hepatoma cells. J Biol Chem 1990;27:16096–16101.
Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ. Plasma antigen levels of the lipoprotein associated coagulation inhibitor in patient samples. Blood 1991;78:387–393.
Kowaka T, Abumiya, Kimura T, et al. Tissue factor pathway inhibitor activity in human plasma. Measurement of lipoprotein associated and free forms in hyperlipidemia. Arterioscl Thromb Vasc Biol 1995;15:504–510.
Shimura M, Wada H, Nakasaki T, et al. Increased truncated form of plasma tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol 1999;60:94–98.
Lupu C, Goodwin CA, Westmuckett AD, et al. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomain caveolae. Regulatory mechanism(s) of the anticoagulant properties of the endothelium. Arterioscler Thromb Vasc Biol 1997;17:2964–2974.
Creasey AA, Chang ACK, Fiegen L, et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 1993;91:2850–2860.
Carr C, Bild GS, Chang ACK, et al. Recombinant E. coli-derived tissue factor pathway inhibitor reduces coagulopathic and lethal effects in the baboon gram-negative model of septic shock. Circ Shock 1995;44:126–137.
Warr TA, Rao LVM, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: Effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood 1990;75:1481–1489.
Sandset PM, Warn-Cramer BJ, Rao LVM, Maki SL, Rapaport SI. Depletion of extrinsic pathway inhibitor (EPI) sensitizes rabbits to disseminated intravascular coagulation induced with tissue factor: Evidence supporting a physiologic role for EPI as a natural anticoagulant. Proc Natl Acad Sci USA 1991;88:708–712.
Sandset PM, Warn-Cramer BJ, Maki SL, Rapaport SI. Immunodepletion of extrinsic pathway inhibitor sensitizes rabbits to endotoxin-induced intravascular coagulation and the generalized Shwartzman reaction. Blood 1991;78:1496–1502.
Osterud B, Flaegstad T. Increased thromboplastin activity in monocytes of patients with meningococcal infection: Related to an unfavorable prognosis. Thromb Haemost 1983; 19:5–7.
Day KC, Hoffman LC, Palmier MO, et al. Recombinant lipoprotein-associated coagulation inhibitor inhibits tissue thromboplastin-induced intravascular coagulation in the rabbit. Blood 1990;76:1538–1545.
Bregengard C, Nordfang O, Wildgoose P, Svendsen O, Hedner V, Diness V. The effect of two-domain tissue factor pathway inhibitor on endotoxin-induced disseminated intravascular coagulation in rabbits. Blood Coag Fibrinol 1993;4:699–706.
Biemond BJ, Levi M, Ten Cate H, et al. Complete inhibition of endotoxin-induced coagulation activation in chimpanzees with a monoclonal Fab fragment against factor VII/VIIa. Thromb Haematol 1995;73:223–230.
Taylor FB, Chang AC, Peer G, et al. Active site inhibited factor VIIa (DEGR VIIA) attenuates the coagulant and interleukin-6 and-8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood 1998;91:1609–1615.
Taylor FB Jr, Chang AC, Peer GT, et al. DEGR-factor Xa blocks disseminated intravascular coagulation initiated by Escherichia coli without preventing shock or organ damage. Blood 1991;78:364–68.
Rapaport, SI. The extrinsic pathway inhibitor: A regulator of tissue factor-dependent blood coagulation. Thromb Haemost 1991;66:6–15.
Taylor FB, Chang A, RufW, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock 1991;33:127–134.
Randolph MM, White GL, Kosanke SD, et al. Attenuation of tissue thrombosis and hemorrhage by ala-TFPI does not account for its protection against E. coli-A comparative study of treated and untreated non-surviving baboons challenged with LD100E. coli. ThrombHaemost 1998;79:1048–1053.
Camerota AJ, Creasey AA, Patla V, Larkin VA, Fink MP. Delayed treatment with recombinant human tissue factor pathway inhibitor improves survival in rabbits with gramnegative peritonitis. J Infect Dis 1998;177:668–676.
Goldfarb RD, Glock D, Johnson K, et al. Randomized, blinded, placebo-controlled trial of tissue factor pathway inhibitor in porcine septic shock. Shock 1998;10:258–264.
Jansen P, van Lopik T, Lubbers YPT, et al. The coagulantinflammatory axis in the baboon response to E. coli: Effects of tissue factor pathway inhibitor on hemostatic balance, the cytokine network and the release of apoptosis marker sFas. In: Inflammatory Mediators in Primate Sepsis. Thesis,Amsterdam; University of Amsterdam 1997:95–107.
Johnson K, Aarden L, Choi Y, De Groot E, Creasey A. The proinflammatory cytokine response to coagulation and endotoxin in whole blood. Blood 1996;87:5051–5060.
Stack AM, Saladino RA, Thompson C, et al. Failure of prophylactic and therapeutic use of a murine anti-tumor necrosis factor monoclonal antibody in E. coli sepsis in the rabbit. Crit Care Med 1995;23:1512–1518.
Matyal R, Vin Y, Delude R, et al. Extremely low doses of tissue factor pathway inhibitor decrease mortality in a rabbit model of septic shock. Submitted??
Park CT, Creasey AA, Wright SD. Tissue factor pathway inhibitor blocks cellular effects of endotoxin by binding to endotoxin and interfering with transfer of CD 14. Blood 1997;89:4268–4272.
Opal SM, Creasey AA, Palardy JE, Parejo N. The activity of recombinant human tissue factor pathway inhibitor in superantigen-induced sepsis in polymicrobial intra-abdominal sepsis. Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, California, September 26–29, 1999.
Broze, GJ Jr. Tissue factor pathway inhibitor gene disruption. Blood Coag Fibrinol 1998;9(1):S89–92.
Braeckman R, Childs A, Creasey AA, Hung D, Guler HP. Pharmacokinetics and pharmacodynamics (PK/PD) of recombinant tissue factor pathway inhibitor (rTFPI) in human volunteers. Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, September 28–October 1, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Creasey, A.A. New Potential Therapeutic Modalities: Tissue Factor Pathway Inhibitor. Sepsis 3, 173–182 (1999). https://doi.org/10.1023/A:1009811819077
Issue Date:
DOI: https://doi.org/10.1023/A:1009811819077