Abstract
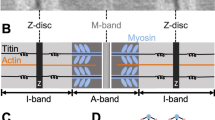
We present a classification of the proteins of the cardiomyocyte based on structural and functional properties of the various components of this cell. The following protein families are categorized: 1) the contractile proteins, responsible for the contractile properties; 2) the sarcomeric skeleton, including titin, α-actinin, myomesin, M-protein, and C-protein, that keeps the contractile filaments in register and ensures sarcomeric stability; 3) the cytoskeletal proteins, i.e., desmin and the microtubules, that maintain the structural order within the cell and connect the cytoplasm and all cellular organelles with the sarcolemma; 4) membrane-associated proteins, such as vinculin, talin, dystrophin, and spectrin, that link the structural components of the intracellular milieu with those of the extracellular matrix via the integrins; and 5) proteins of the intercalated disc, including the cadherins, catenins, desmoplakin, connexin 43, and several others, that ensure stability of the longitudinal cardiomyocyte connections and facilitate impulse conduction.
This classification not only is useful from a structural point of view but also is reflected in the functional behavior of these proteins in different pathophysiological situations, e.g., acute ischemia or chronic damage such as heart failure. Structural alterations, as shown here in human myocardium with chronic heart failure, demonstrate a graded sensitivity to pathophysiologic stimuli in that the contractile proteins are the most sensitive proteins and the cytoskeleton and the membrane-associated proteins show a compensatory increase and are more resistant against noxious stimuli. From these findings, it is concluded that these reactions to degenerative chronic processes reflect the survival priorities of the cells.
Similar content being viewed by others
References
Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg 1995;110: 89–98.
Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultras-tructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 1991;83:504–514.
Hein S, Scholz D, Fujitani N, Rennollet H, Brand T, Friedl A, Schaper J. Altered expression of titin and contractile proteins in failing human myocardium. J Mol Cell Cardiol 1994;26:1291–1306.
Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 1996;76:371–423.
Blanchard A, Ohanian V, Critchley D. Thestructure and func-tion of a-actinin. J Muscle Res Cell Motil 1989;10:280–289.
Beggs AH, Byers TJ, Knoll JHM, Boyce FM, Bruns GAP, Kunkel LM. Cloning and characterization of two human skeletal muscle a-actinin genes located on chromosomes 1 and 11. J Biol Chem 1992;267:9281–9288.
Burridge K, Ferramisco JR. Non-muscle a-actinins are cal-cium sensitive actin binding proteins. Nature 1981;294:565–567.
Sanger JM, Mittal B, Pochapin MB, Sanger JW. Myofibrillo-genesis in living cells microinjected with fluorescently la-beled alpha-actinin. J Cell Biol 1986;102:2053–2066.
Goebel HH, Piirsoo A, Warlo I, Schofer O, Kehr S, Gaude M. Infantile intranuclear rod myopathy. J Child Neurol 1997; 12:22–30.
Fürst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten non-repetitive epitopes starting at the Z line extends close to the M line. J Cell Biol 1988;106:1563–1572.
Fürst DO, Nave R, Osborn M, Weber K. Repetitive titin epitopes with a 42 nm spacing coincide in relative position with known A band striations are identified by major myosin-associated proteins. An immunoelectron-micro-scopical study on myofibrils. J Cell Sci 1989;94:517–527.
Labeit S, Gautel M, Lakey A, Trinick J. Towards a molecular understanding of titin. EMBO J 1992;11:1711–1716.
Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology [see comments]. Circ Res 1997;80:290–294.
Fürst DO, Gautel M. The anatomy of a molecular giant: how the sarcomere cytoskeleton is assembled from immuno-globulin superfamily molecules. J Mol Cell Cardiol 1995; 27:951–959.
Yajima H, Ohtsuka H, Kawamura Y, Kume H, Murayama T, Abe H, Kimura S, Maruyama K. A 11.5 kb 5'terminal cDNA sequence of chicken breast muscle connectin/titin reveals its Z-line binding region. Biochem Biophys Res Commun 1996; 223:160–164.
Gautel M, Goulding D, Bullard B, Weber K, Fürst DO. The central disk region of titin is assembled from a novel repeat in variable copy numbers. J Cell Sci 1996;109:2747–2754.
Helmes M, Trombitas K, Granzier H. Titin develops re-storing force in rat cardiac myocytes. Circ Res 1996;79:619–626.
Trombitas K, Jin JP, Granzier H. The mechanically active domain of titin in cardiac muscle. Circ Res 1995;77:856–861.
Isaacs WB, Kim IS, Struve A, Fulton AB. Biosynthesis of titin in cultured skeletal muscle cells. J Cell Biol 1989; 109:2189–2195.
Hein S, et al. The role of alpha-actinin, titin, and myomesin for the sarcomerogenesis in cultured adult rat myocytes and in failing human hearts. Circulation 1993;:2911.
Eppenberger HM, Perriard JC, Rosenberg UB, Strehler EE. The Mr 165,000 M-protein myomesin: a specific protein of cross striated muscle cells. J Cell Biol 1981;89:185–193.
Grove BK, Cerny L, Perriard JC, Eppenberger HM. Myo-mesin and M-protein: expression of two M-band proteins in pectoral muscle and heart during development. J Cell Biol 1985;101:1413–1421.
Obermann W, Plessmann U, Weber K, Fürst DO. Purifica-tion and biochemical characterization of myomesin, a myosin and titin binding protein from bovine skeletal muscle. Eur J Biochem 1995;208:110–115.
Obermann WMJ, Gautel M, Steiner F, van der Veen PFM, Weber K, Fürst DO. The structure of the sarcomeric M-band: localization of defined domains of myomesin, M-pro-tein, and the 250-kD carboxyterminal region of titin by im-munoelectron microscopy. J Cell Biol 1996;134:1441–1453.
Obermann WMJ, Gautel M, Weber K, Fürst DO. Molecular structure of the sarcomeric M-band: mapping of titin and myosin binding domains in myomesin and the identification of a potential phosphorylation site in myomesin. EMBO J 1997;16:211–220.
Carlsson E, Grove BK, Wallimann HM, Eppenberger HM, Thornell LE. Myofibrillar M-band proteins in rat skeletal muscles during development. Histochemistry 1990;95:27–35.
Einheber S, Fishman DA. Isolation and characterization of a cDNA clone encoding avian skeletal muscle C-protein: an intracellular member of the immunoglobulin super family. Proc Natl Acad Sci USA 1990;87:2157–2161.
Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hain-que B, Gautel M, Labeit S, James M, Beckmann J, Weissen-bach J, Vosberg HP, Fiszman M, Komajda M, Schwartz K. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardio-myopathy. Nature Genet 1995;11:438–440.
Traub P. Intermediate Filaments. Berlin, Heidelberg, New York, Tokyo: Springer, 1985.
Bloemendal H, Raats JMH, Pieper FR, Benedetti EL, Dunia I. Transgenic mice carrying chimeric or mutated type III intermediate filament (IF) genes. CMLS Cell Mol Life Sci 1997;53:1–12.
Klymkowsky MW. Intermediate filaments: new proteins, some answers, more questions. Curr Opin Cell Biol 1995;7: 46–54.
Li Z, Colucci E, Babinet C, Paulin D. The human desmin gene: a specific regulatory program in skeletal muscle both in vitro and in transgenic mice. Neuromusc Disorders 1993; 3(5–6):423–427.
Weitzer G, Milner DJ, Kim JU, Bradley A, Capetanaki Y. Cytoskeletal control of myogenesis: a desmin null mutation blocks the myogenic pathway during embryonic stem cell differentiation. Dev Biol 1995;172:422–439.
Capetanaki Y, Milner DJ, Weitzer G. Desmin in muscle for-mation and maintenance: knockouts and consequences. Cell Struct Funct 1997;22(1):103–116.
Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degenera-tion in mice lacking desmin. J Cell Biol 1996;134:1255–1270.
Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myo-blast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol 1994;124:827–841.
Zachara E, Bertini E, Lioy E, Boldrini R, Prati PL, Bosman C. Restrictive cardiomyopathy due to desmin accumulation in a family with autosomal dominant inheritance. Giornale Italiano di Cardiologia 1997;27:436–442.
Kim HD. Expression of intermediate filament desmin and vimentin in the human fetal heart. Anat Record 1996;246: 271–278.
Nogales E, Wolf SG, Downing KH. Structure of the al-pha-beta tubulin dimer by electron crystallography. Nature 1998;391:199–203.
Cleveland D. The multi tubuli hypothesis revisited: what have we learned? J Cell Biol 1987;104:381–383.
Mandelkow E, Mandelkow EM. Microtubules and micro-tubule associated proteins. Curr Opin Cell Biol 1995;7: 72–81.
Samuel JL, Bertier B, Bugaisky L, Marotte F, Swynghedauw B, Schwartz K, Rapaport L. Different distri-butions of microtubules, desmin filaments and isomyosins during the onset of cardiac hypertrophy in the rat. Eur J Cell Biol 1984;34:300–306.
Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role in the contractile dysfunction in hypertrophied cardiocytes. Sci-ence 1993;260:682–687.
Tagawa H, Rozich JD, Tsutsui H, Narashige T, Kup-puswamy D, Sato H, McDermott PJ, Koide M, Cooper G. Basis for increased microtubules in pressure-hypertrophied cardiocytes. Circulation 1996;93:1230–1243.
Ervasti JM, Kahl SD, Campbell KP. Purification of dystro-phin from skeletal muscle. J Biol Chem 1991;266:9161–9165.
Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol 1993;5:82–87.
Klietsch L, Ervasti J, Arnold W, Campbell KP, Jorgensen AO. Dystrophin-glycoprotein complex and laminin colocal-ize to the sarcolemma and the transverse tubules of cardiac muscle. Circ Res 72 1993;349–360.
Ohlendieck K. Towards an understanding of the dystro-phin-glycoprotein complex: linkage between the extracellu-lar matrix and the membrane cytoskeleton in muscle fibers. Eur J Cell Biol 1996;69:1–10.
Yarom R, Morris GE, Froede R, Schaper J. Myocardial dystrophin immunolocalization at sarcolemma and trans-verse tubules. Experientia 1992;48:614–616.
Bies RD, Friedman D, Roberts R, Perryman MB, Caskey CT. Expression and localization of dystrophin in human car-diac Purkinje fibers. Circulation 1992;86:147–153.
Menkel AR, Kroemker M, Bubeck P, Ronsiek M, Nikolai G, Jockosch BM. Characterization of an actin binding domain in the cytoskeletal protein vinculin. J Cell Biol 1994;1231–1240.
Jones P, Jackson P, Price GJ, Patel B, Ohanion V, Lear AL, Critchley DR. Identification of a talin binding site in the cy-toskeletal protein vinculin. J Cell Biol 1989;109:2917–2927.
Johnson RP, Craig SW. An intramolecular association be-tween the head and tail domains of vinculin modulates talin binding. J Biol Chem 1994;269:12611–12619.
Otto J. Vinculin and reviews. Cell Motil Cytoskeleton 1990; 16:1–6.
Burridge K, Mangeat P. An interaction between vinculin and talin. Nature 1984;308:744–748.
Burridge K, Fath K, Kelly T, Nuckols G, Turner C. Focal adhesions: transmembrane junctions between the extracel-lular matrix and the cytoskeleton. Annu Rev Cell Biol 1988; 4:487–525.
Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. BioEssays 1989;10:104–108.
Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Div 1996;12: 463–519.
Beckerle MC, Yeh RK. Talin: role and sites of cell-substra-tum adhesion. Views and reviews. Cell Motil Cytoskeleton 1990;16:7–13.
Rees DJG, Ades SE, Singer SJ, Hynes RO. Sequence and domain structure of talin. Nature 1990;347:685–687.
Belkin AM, Zhidkova NI, Koteliansky VE. Localization of talin in skeletal and cardiac muscles. FEBS 1986;200:32–36.
Drenckhahn D, Beckerle M, Burridge K, Otto J. Identifica-tion and subcellular location of talin in various cell types and tissues by means of [125I]vinculin overlay, immunoblotting and immunocytochemistry. Eur J Cell Biol 1988;46:513–522.
Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junctional protein from liver. J Cell Biol 1987;105:2621–2629.
Severs NJ. Review. The cardiac gap junction and interca-lated disc. Int J Cardiol 1990;26:136–173.
Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the connexin43 gap-junction gene in pa-tients with heart malformations and defects of laterality. N Engl J Med 1995;332:1323–1329.
Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 1994;90:713–725.
Takeichi M. Cadherin cell adhesion receptors as a morpho-genetic regulator. Science 1991;251:1451–1455.
Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr Opin Cell Biol 1994;6:682–687.
Hertig CM, Eppenberger-Eberhard M, Koch S, Eppenber-ger HM. N-cadherin in adult rat cardiomyocytes in culture. I. Functional role of N-cadherin and impairment of cell-cell contact by truncated N-cadherin mutant. J Cell Sci 1996; 109:1–10.
Goncharova EJ, Kam Z, Geiger B. The involvement of ad-herens junction components in myofibrillogenesis in cul-tured cardiac myocytes. Development 1992;114:173–183.
Kostin S, Hein S, Maeno Y, Scholz D, Schaper W, Schaper J. Spatio-temporal distribution of intercellular junctions in adult rat cardiomyocytes in culture (abstract). Circulation 1997;96:4145.
Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci 1996;109:11–20.
Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989;8:1711–1717.
Nagafuchi A, Takeichi M, Tsukida S. The 102 Kd cadherin-associated protein similarity to vinculin and posttranscrip-tional regulation of expression. Cell 1991;65:849–857.
Knudsen KA, Wheelock MJ. Plakoglobin, or an 83 Kd homo-logue distinct from b-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol 1992;118:671–679.
Mueller H, Franke WW. Biochemical and immunological characterization of desmoplakins I and II, the major poly-peptides of the desmosomal plaque. J Mol Biol 1983;163: 647–671.
Angst BD, Nilles LA, Green KJ. Desmoplakin II expression is not restricted to stratified epithelia. J Cell Sci 1990;97: 247–257.
Virata MLA, Wagner RM, Parry DAD, Green KJ. Molecular structure of the human desmoplakin I and II amino termi-nus. Proc Natl Acad Sci USA 1992;89:544–548.
Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces dis-rupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol 1996;134:985–1001.
Sorimachi H, Freiburg A, Kolmerer B, Ishiura S, Stier G, Gregorio CC, Labeit D, Linke WA, Suzuki K, Labeit S. Tissue-specific expression and a-actinin binding properties of the Z-disc titin: implications for the nature of the verte-brate Z-discs. J Mol Biol 1997;270:688–695.
Rights and permissions
About this article
Cite this article
Kostin, S., Heling, A., Hein, S. et al. The Protein Composition of the Normal and Diseased Cardiac Myocyte. Heart Fail Rev 2, 245–260 (1998). https://doi.org/10.1023/A:1009797831654
Issue Date:
DOI: https://doi.org/10.1023/A:1009797831654