Abstract
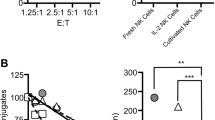
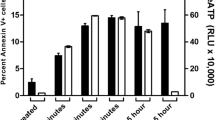
We and others have recently shown that human NK cells express the Fas ligand (FasL) constitutively and that they can trigger the lysis of Fas positive (Fas+) target cells (TC) by apoptosis. We have also previously demonstrated that NK cells exposed to sensitive TC temporarily lose their ability to lyse sensitive TC via the granule-mediated pathway and that this loss is recovered when inactivated NK cells (NKi) are incubated in medium supplemented with IL-2, IL-12 or IL-15. In this study, we investigated the fate of the Fas-lytic pathway in NK cells exposed to either Fas+ or Fas− TC. To this end, we exposed NK cells to Jurkat (Fas−) or Jurkat (Fas+) TC for up to 6 h, separated NK cells from the TC and assessed the residual lytic activity against K562, a traditional human NK cell target, Jurkat Fas+ and Jurkat Fas− TC. Fas lytic activity was determined in calcium free medium, in the presence or absence of two distinct Fas-blocking monoclonal antibodies and a Fas.Fc fusion protein. In parallel experiments, the extent of DNA fragmentation in the three TCs was also assayed by the JAM test. Our results indicate that: (i) NK cells exposed to susceptible Fas+ TC temporarily lose most of their lytic potential due to the granule-mediated pathway, while only partially losing the Fas-lytic pathway. They also partially lose their ability to fragment DNA. (ii) NK cells exposed to Fas+ TC completely recover the Fas lytic pathway and the ability to fragment DNA via the Fas/Fas ligand when incubated in medium supplemented with IL-2 for 18 h.
Similar content being viewed by others
References
Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood 1990; 79: 2421–2438.
Moretta A, Biassoni R, Bottino C, et al. Major histocompatibility complex, Class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev 1997; 155: 105–117.
Raulet D, Held W. Natural killer cell recepcors: The offs and ons of NC cell recognition. Cell 1995; 82: 697–700.
Lopez-Botet M, Moretta L, Stominger J. NK-cell receptors and recognition of MHC Class I molecules. Immunol Today 1996; 17: 212–214.
Krahenbuhl O, Tschopp J. Involvement of granule proteins in T-cell-mediated lysis. Nat Immunol Cell Growth Regul 1990; 9:274–282.
Liu C-C, Walsh CM, Young JD-E. Perform structure and function. Immunol Today 1995; 16: 194–201.
Montel AH, Bochan MR, Hobbs JA, et al. Fas involvement in cytotoxicity mediated by NK cells. Cell Immunol 1995; 166: 236–246.
Oshimi Y, Oda S, Honda Y, et al. Involvement of Fas ligand and Fas mediated pathway in the cytotoxicity of human natural killer cells. J Immunol 1996; 157: 2909–2915.
Eischen CM, Schilling JD, Lynch DH, et al. Fc receptor induced expression of Fas-ligand and activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J lmmunol 1996; 156: 2693–2699.
Nagata S, Goldstein P. The Fas death factor. Science 1995; 267: 1449–1456.
Suda T, Takahashi T, Goldstein P, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis receptor family. Cell 1993; 75: 1169–1178.
McGahon A, Nishioka W, Martin S, et al. Regulation of the Fas apoptotic cell death pathway by Abl. J Biol Chem 1995; 270: 22625–22631.
Kagi D, Vignaux F, Ledermann B, et al. Fas and perforin pathways as major mechanisms of T cell-medicated cytotoxicity. Science 1994; 265: 528–530.
Lowin B, Kajne M, Mattman C, et al. Cytolytic T-cells cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994; 370: 650–652.
Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev 1995; 146: 57–59.
Isaaz S, Baetz K, Olsen K, et al. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. E J Immunol 1995; 25: 1071–1079.
Heusel J, Wesselschmidt R, Shresta S, et al. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994; 76: 977–987.
Bellgrau D, Gold D, Selawry H, et al. A role for CD95 ligand in preventing graft rejection. Nature 1995; 377: 630–632.
Strassner A. Death of a T cell. Nature 1995; 373: 385–386.
Rieux-Laucat F, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995; 268: 1347–1349.
Abrams S I, Brahmi Z. Target cell directed NK inactivation. Concomitant loss of NK and antibody dependent cellular cytotoxicity activities. J Immunol 1988; 140: 2090–2095.
Hommel-Berrey GA, Shenoy AM, Brahmi Z. Receptor modulation and early signal transduction events in cytotoxic T lymphocytes inactivated by sensitive target cells. J Immunol 1991; 147: 3237–3243.
Bajpai A, Kwon BS, Brahmi Z. Rapid loss of perforin and serine protease RNA in cytotoxic lymphocytes exposed to sensitive target cells. Immunology 1991; 74: 258–264.
Bochan MR, Brahmi Z. Target cell-directed rapid degradation of TNF-α messenger RNA in human cytotoxic T cells. Immunol Lett 1994; 40: 37–42.
Goebel S, Schloemer R, Brahmi Z. Target cell-induced perforin mRNA turnover in NK3.3 cells is mediated by multiple elements within the mRNA coding region. Molecular Immunol 1996; 33: 341–349.
Kung S, Miller R. Mouse natural killer subsets defined by their target specificity and their ability to be separately rendered unresponsive in vivo. J Immunol 1997; 158: 2616–2626.
Dhein J, Waiczak H, Baumler C, et al. Autocrin T-cell suicide mediated by APO-I (Fas/CD95). Nature 1995; 373: 438–441.
Matzinger P. The JAM test: a simple assay for DNA fragmentation and cell death. J Immunol Methods 1991; 145: 185.
Abrams SI, Brahmi Z. The functional loss of human natural killer cell activity induced by K562 is reversible via an interleukin-2 dependent mechanism. Cell Immunol 1986; 101: 558–570.
Reiner NE. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today 1994; 8: 374–381.
Brahmi Z, Bray RA, Abrams SI. Evidence for an early calcium independent event in the inactivation of the natural killer cell cytotoxic mechanism. J Immunol 1985; 135: 4108–4113.
Perussia B, Trinchieri G. Inactivation of natural killer cell cytotoxic activity after interaction with target cells. J Immunol 1981; 126: 754–758.
Heiskala MK, Carpen O, Saksela E. Mechanism of cell contact mediated inhibition of natural killer cell activity. J Immunol 1987; 139: 1414–1418.
Bajpai A, Brahmi Z. Target cell induced inactivation of cytolytic lymphocytes. Role and regulation of CD45 and calyculin A-inhibited phosphatase in response to interleukin-2. J Biochem 1994; 269: 18864–18869.
Bonavida B, Lebow LT, Jewett A. Natural killer cell subsets: maturation, differentiation and regulation. Nat Immunity 1993; 12: 194–208.
Rouvier E, Luciani MF, Goldstein P. Fas involvement in Ca2+independent T cell-mediated cytotoxicity. J Exp Med 1993; 177: 195–200.
O'Connell J, O'Sullivan G.C., Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1997; 184: 1075–1081.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Csipo, I., Montel, A.H., Hobbs, J.A. et al. Effect of Fas+ and Fas− Target Cells on the Ability of NK Cells to Repeatedly Fragment DNA and Trigger Lysis via the Fas Lytic Pathway. Apoptosis 3, 105–114 (1998). https://doi.org/10.1023/A:1009696908600
Issue Date:
DOI: https://doi.org/10.1023/A:1009696908600