Abstract
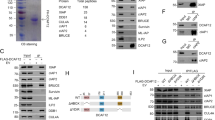
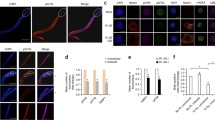
Several reports describing Daxx and its putative role have emerged without a unifying theme. While Daxx has been implicated in apoptosis, it remains unclear whether Daxx is pro- or anti-apoptotic, and whether its role in apoptosis is direct or indirect. Moreover, whether Daxx plays alternative or additional roles in regulating transcription, centromere binding or any number of other activities within the cell, is uncertain. The ability of Daxx to interact with a wide variety of molecules in yeast-interaction trap systems (Table 1) has allowed for this range of speculation. The fact that Daxx contains no significant homology to other known proteins has rendered its study all the more challenging.
Similar content being viewed by others
References
Kiriakidou M, Driscoll DA, Lopez-Guisa JM, Strauss III JF. Cloning and expression of primate Daxx cDNAs and mapping of the human gene to chromosome 6p21.3 in the MHC region. DNA Cell Biol 1997; 16:1289–1298.
Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 1997; 89: 1067–1076.
Pluta AF, Earnshaw WC, Goldberg IG. Interphase-specific association of intrinsic centromere protein CENP-C with HDaxx, a death domain-binding protein implicated in Fas-mediated cell death. J Cell Sci 1998; 111: 2029–2041.
Hollenbach AD, Sublett JE, McPherson CJ, Grosveld G. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. Embo J 1999; 18: 3702–3711.
Michaelson JS, Bader D, Kuo F, Kozak C, Leder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev 1999; 13: 1918–1923.
Chang HY, Yang X, Baltimore D. Dissecting Fas signaling with an altered-specificity death-domain mutant: Requirement of FADD binding for apoptosis but not Jun N-terminal kinase activation. Proc Natl Acad Sci USA 1999; 96: 1252–1256.
Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998; 281: 1860–1863.
Ishov AM, Sotnikov AG, Negorev D, et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol 1999; 147: 221–234.
Torii S, Egan DA, Evans RA, Reed JC. Human Daxx regulates fas-induced apoptosis from nuclear PML oncogenic domains (PODs) [In Process Citation]. Embo J 1999; 18: 6037–6049.
Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol 1998; 8: 1001–1008.
Yeh WC, Pompa JL, McCurrach ME, et al. FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998; 279: 1954–1958.
Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 1998; 392: 296–300.
Everett RD, Earnshaw WC, Pluta AF, et al. A dynamic connection between centromeres and ND10 proteins. J Cell Sci1999; 112: 3443–3454.
Lei H, Oh SP, Okano M, et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development 1996; 122: 3195–3205.
Panning B, Jaenisch R. DNA hypomethylation can activate Xist expression and silence X-linked genes. Genes Dev 1996; 10: 1991–2002.
de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 1990; 347: 558–561.
Kakizuka A, Miller WH, Jr., Umesono K, et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 1991; 66: 663–674.
Dyck JA, Maul GG Miller WH, Jr., Chen JD, Kakizuka A, Evans RM. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 1994; 76: 333–343.
Wang ZG, Ruggero D, Ronchetti S, et al. PML is essential for multiple apoptotic pathways. Nat Genet 1998; 20: 266–272.
Quignon F, De Bels F, Koken M, Feunteun J, Ameisen JC, de The H. PML induces a novel caspase-independent death process. Nat Genet 1998; 20: 259–265.
Varfolomeev EE, Schuchmann M, Luria V, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9: 267–276.
Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet 1995; 11: 294–300.
Adachi M, Suematsu S, Suda T, et al. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci USA 1996; 93: 2131–2136.
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998; 8: 297–303.
LaMorte VJ, Dyck JA, Ochs RL, Evans RM. Localization of nascent RNA and CREB binding protein with the PMLcontaining nuclear body. Proc Natl Acad Sci USA 1998; 95: 4991–4996.
Doucas V, Tini M, Egan DA, Evans RM. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc Natl Acad Sci USA 1999; 96: 2627–2632.