Abstract
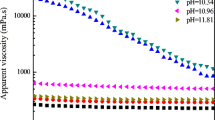
Dolomite failed to precipitate despite more than1000-fold oversaturation (IAP approximately10-13.8) from dilute solution (ionic strength =0.025) at 25 °C after 32 years.
Similar content being viewed by others
References
Arvidson, R. S. and Mackenzie, F. T. (1997) Tentative kinetic model for dolomite precipitation rate and its application to dolomite distribution. Aquatic Geochemistry 2, 273–298.
Barnes, I. and Back, W. (1964) Dolomite solubility in ground water. U.S. Geological Survey Prof. Paper 475-D 160, 179–180.
Chave, K. E. (1952) A solid solution between calcite and dolomite. J. Geology 60, 190–192.
Folk, R. L. (1993) SEM imaging of bacteria and nannobacteria in carbonate sediments and rocks. J. Sed. Petrology 63, 990–999.
Helgeson, H. C., Delany, J. M., Nesbitt, H. W., and Bird, D. K. (1978) Summary and critique of the thermodynamic properties of rock-forming minerals. Amer. J. Sci. 278-A, 229 pp.
Holland, H. D., Kirssipu, T. V., Huebner, J. S., and Oxburg, U. M. (1964) On some aspects of the chemical evolution of cave waters. J. Geology 72, 36–67.
Hsü, K. J. (1963) Solubility of dolomite and composition of Florida ground waters. J. Hydrology 1, 288–310.
Land, L. S. (1967) Diagenesis of skeletal carbonates. J. Sed. Petrology 37, 914–930.
Land, L. S. and Epstein, S. (1970) Late Pleistocene diagenesis and dolomitization, North Jamaica. Sedimentology 14, 184–200.
Langmuir, D. (1971) The geochemistry of some carbonate ground waters in central Pennsylvania. Geochim. Cosmochim. Acta 35, 1023–1045.
Miser, D. E. (1987) Microstructures in Natural and Synthetic Dolomite. Unpublished PhD Dissertation, University of Texas, Austin, 327 pp.
Mitchell, J. T., Land, L. S., and Miser, D. E. (1987) Modern marine dolomite cement in a north Jamaican fringing reef. Geology 15, 557–560.
Morse, J. W. and Mackenzie, F. T. (1990) Geochemistry of Sedimentary Carbonates. Elsevier, Amsterdam, 707 pp.
Parkhurst, D L., Thorstenson, D. C., and Plummer, L. N. (1980) PHREEQE - A computer program for geochemical calculations. U.S. Geol. Survey Water-Resources Investigations 80-96, 159 pp.
Rosenberg, P. E. and Holland, H. D. (1964) Calcite-dolomite-magnesite stability relations in solutions at elevated temperatures. Science 145, 700–701.
Sibley, D. F., Nordeng, S. H., and Borkowski, M. L. (1994) Dolomitization kinetics in hydrothermal bombs and natural settings. J. Sed. Research A64, 630–637.
Vasconcelos, C. and McKenzie, J. A. (1997) Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio De Janeiro, Brazil). J. Sed. Research 67, 378–390.
Rights and permissions
About this article
Cite this article
Land, L.S. Failure to Precipitate Dolomite at 25 °C fromDilute Solution Despite 1000-Fold Oversaturation after32 Years. Aquatic Geochemistry 4, 361–368 (1998). https://doi.org/10.1023/A:1009688315854
Issue Date:
DOI: https://doi.org/10.1023/A:1009688315854