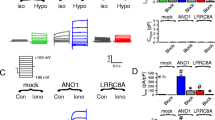
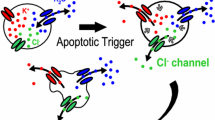
Abstract
To investigate the involvement of K+ efflux in apoptotic cell shrinkage, we monitored efflux of the K+ congener,86 Rb+, and cell volume during CD95-mediated apoptosis in Jurkat cells. An anti-CD95 antibody caused apoptosis associated with intracellular GSH depletion, a significant increase in 86Rb+ efflux, and a decrease in cell volume compared with control cells. Preincubating Jurkat cells with Val-Ala-Asp-chloromethylketone (VAD-cmk), an inhibitor of caspase proteases, prevented the observed 86Rb+ efflux and cell shrinkage induced by the anti- CD95 antibody. A wide range of inhibitors against most types of K+ channels could not inhibit CD95-mediated efflux of86 Rb+, however, the uptake of86 Rb+ by Jurkat cells was severely compromised when treated with anti-CD95 antibody. Uptake of86 Rb+ in Jurkat cells was sensitive to ouabain (a specific Na+/K+-ATPase inhibitor), demonstrating Na+/K+-ATPase dependent K+ uptake. Ouabain induced significant86 Rb+ efflux in untreated cells, as well as it seemed to compete with86 Rb+ efflux induced by the anti-CD95 antibody, supporting a role for Na+/K+-ATPase in the CD95-mediated86 Rb+ efflux. Ouabain treatment of Jurkat cells did not cause a reduction in cell volume, although together with the anti-CD95 antibody, ouabain potentiated CD95-mediated cell shrinkage. This suggests that the observed inhibition of Na++/K+-ATPase during apoptosis may also facilitate apoptotic cell shrinkage.
Similar content being viewed by others
References
Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Rasgado-Flores H, Pena-Rasgado C, Ehrenpreis S. Cell volume and drug action: some interactions and perspectives. Drug Develop Res 1995; 36: 61–80.
H¨aussinger D. The role of cellular hydration in the regulation of cell function. Biochem J 1996; 313: 697–710.
Bortner CD, Hughes FM Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 1997; 272: 32436–32442.
Hughes FM Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 1997; 272: 30567–30576.
Hoffman EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev 1989; 69: 315–382.
Barbiero G, Duranti F, Bonelli G, Amenta JS, Baccino FM. Intracellular ionic variations in the apoptotic death of L cells by inhibitors of cell cycle progression. Exp Cell Res 1995; 217: 410–418.
Benson RSP, Heer S, Dive C, Watson JM. Characterization of cell volume loss in CEM-C7 A cells during dexamethasoneinduced apoptosis. Am J Physiol 1996; 270(4 Pt1): C1190–C1203.
McCarthy JV, Cotter TG. Cell shrinkage and apoptosis: a role for potassium and sodium efflux. Cell Death Differ 1997; 4: 756–770.
Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr 1992; 24: 249–261.
Tosteson DC, Hoffman JF. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol 1960; 44: 169–194.
MacKnight ADC, Leaf A. Regulation of cellular volume. Physiol Rev 1997; 57: 510–573.
Alvarez-Leefmans FJ, Gamiño SM, Reuss L. Cell volume changes upon sodium pump inhibition in helix aspersa neurones. J Physiol 1992; 458: 603–619.
Smith TW, Rasmusson RL, Lobaugh LA, Lieberman M. Na+/K+pump inhibition induces cell shrinkage in cultured chick cardiac myocytes. Basic Res Cardiol 1993; 88: 411–420.
van den Dobbelsteen DJ, Nobel CSI, Schlegel J, Cotgreave IA, Orrenius S, Slater AFG. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem 1996; 271: 15420–15427.
Boon NA, Oh VMS, Taylor EA, Johansen T, Aronson JK, Grahame-Smith DG. Measurement of specific [3H]-ouabain binding to different types of human leucocytes. Br J Clin Pharmacol1984; 18: 153–161.
Nobel CSI, Burgess DH, Zhivotovsky B, Burkitt MJ, Orrenius S, Slater AFG. Mechanism of dithiocarbamate inhibition of apoptosis: thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem Res Toxicol 1997; 10: 636–643.
Celsi G, Nishi A, Akusj¨arvi G, Aperia A. Abundance of Na(+)-K(+)-ATPase mRNA is regulated by glucocorticoid hormones in infant rat kidneys. Am J Physiol 1991; 260(2 Pt2): F192–F197.
Esmann, M. ATPase and phosphatase activity of Na+, K+-ATPase: molar and specific activity, protein determination. Meth Enzymol 1988; 156: 105–115.
Dolle RE, Hoyer D, Prasad CVC, Schmidt SJ, Helaszek CT, Miller RE, Ator MA. P1 aspartate-based peptide alpha-((2,6–dichlorobenzoyl)oxy)methyl ketones as potent time-dependent inhibitors of interleukin-1 beta-converting enzyme. J Med Chem1994; 37: 563–564.
Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 1997; 272: 17907–17911.
Vanags DM, P¨orn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem 1996; 271: 31075–31085.
Cook NS, Quast U. Potassium Channels: Structure, Classification, Function and Theurapeutic Potential. Chichester: Ellis Horwood Ltd., 1989: 181–255.
Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Ann Rev Immunol 1995; 13: 623–653.
Grinstein S, Smith JD. Calcium-independent cell volume regulation in human lymphocytes. Inhibition by charybdotoxin. J Gen Physiol 1990; 95: 97–120.
Beauvais F, Michel L, Dubertret L. Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. role of K+channels. J Leukocyte Biol 1995; 57: 851–855.
Garay RP, Nazaret C, Hannaert PA, Cragoe EJ Jr. Demonstration of a [K+,Cl¡ ]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl¡ ]-cotransport system. Mol Pharmacol 1988, 33: 696–701.
Giunta C, Cavaletto M, Pergola L, Pessione E, Bracchino P. Modulation of Na+/K+pump in intact erythrocytes by cardioglycosides, steroid hormones and ouabain-like compounds. Gen. Parmacol. 1992; 23: 683–687.
Krammer PH. The CD95 (APO-1/Fas) receptor/ligand system: death signals and diseases. Cell Death Differ 1996; 3: 159–160.
Dewitt LM, Putney JW Jr. Alpha-adrenergic stimulation of potassium efflux in guinea-pig hepatocytes may involve calcium influx and calcium release. J Physiol 1984; 346: 395–407.
Bolton TB, Clapp LH. The diverse effects of noradrenaline and other stimulants on 86Rb and 42K efflux in rabbit and guineapig arterial muscle. J Physiol 1984; 355: 43–63.
Castle NA, Haylett DG. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol 1987; 383: 31–43.
Ashcroft FM, Kakei M, Kelly RP. Rubidium and sodium permeability of the ATP-sensitive K+ channel in single rat pancreatic beta-cells. J Physiol 1989; 408: 413–429.
Bortner CD, Cidlowski JA. Caspase independent/dependent regulation ofK+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J Biol Chem 1999; 274: 21953–21962.
H¨aussinger D, Lang F, Bauers K, Gerok W. Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem 1990; 188: 689–695.
van den Dobbelsteen DJ, Nobel CSI, Samuelsson A, Orrenius S, Slater AFG. Glutathione metabolism during apoptosis. In: Montagnier L, Olivier R, Pasquier C, eds. Oxidative Stress, Cancer, AIDS and Neurodegenerative Diseases. New York: Marcel Dekker, 1997: 179–189.
Lang F, Szabo I, Lepple-Wienhues A, Ritter M, Waldegger S, Gulbins E. Cell volume in the regulation of metabolism, cell proliferation and apoptotic cell death. In: Okada Y, ed. Cell Volume Regulation: The Molecular Mechanism and Volume Sensing Machinery. Elsevier Science B.V., 1998: 49–56.
Priestland RN, Whittam R. The temperature dependence of activation by phosphatidylserine of the sodium pump adenosine triphospatase. J Physiol 1972: 220: 353–361.
McConkey DJ, Zhivotovsky B, Orrenius S. Apoptosis-molecular mechanisms and biochemical implications. Molecular Aspects of Medicine 1996; 17: 1–110.
Bertorello AM, Katz AI. Short-term regulation of renal Na-KATPase activity: physiological relevance and cellular mechanisms. Am J Physiol 1993; 265(6 Pt2): F743–F755.
Mashima T, Naito M, Fujita N, Noguchi K, Tsuruo T. Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16–induced U937 apoptosis. Biochem Biophys Res Commun 1995; 217: 1185–1192.
Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol 1990; 110: 349–357.
Larson M, Spring KR. In: Gilles R, Bolls L, Kleinzeller A, eds. Cell Volume Control: Fundamental and Comparative Aspects in Animal Cells. London: Academic Press Inc. Ltd., 1987: 105–123.
Meisenholder GW, Martin SJ, Green DR, Nordberg J, Babior BM, Gottlieb RA. Events in apoptosis. Acidification is downstream of protease activation and Bcl-2 protection. J Biol Chem 1996; 271: 16260–16262.
Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Kufe D. Proteolytic activation protein kinase C ± by an ICE-like protease in apoptotic cells. EMBO J 1995; 14: 6148–6156.
Datta R, Kojima H, Yoshida K, Kufe D. Caspase-3–mediated cleavage of protein kinase C µ in induction of apoptosis. J Biol Chem 1997; 272: 20317–20320.
Gilbert M, Knox S. Influence of Bcl-2 overexpression on Na+/K(+)-ATPase pump activity: correlation with radiationinduced programmed cell death. J Cell Physiol 1997; 171: 299–304.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nobel, C.S.I., Aronson, J.K., van den Dobbelsteen, D.J. et al. Inhibition of Na+/K+-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis 5, 153–163 (2000). https://doi.org/10.1023/A:1009684713784
Issue Date:
DOI: https://doi.org/10.1023/A:1009684713784