Abstract
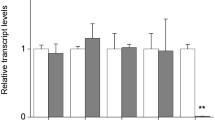
Proteinase inhibitors have been proposed to function as plant defence agents against herbivorous pests. We have introduced the barley trypsin inhibitor CMe (BTI-CMe) into wheat (Triticum aestivum L.) by biolistic bombardment of cultured immature embryos. Of the 30 independent transgenic wheat lines selected, 16 expressed BTI-CMe. BTI-CMe was properly transcribed and translated as indicated by northern and western blot, with a level of expression in transgenic wheat seeds up to 1.1% of total extracted protein. No expression was detected in untransformed wheat seeds. Functional integrity of BTI-CMe was confirmed by trypsin inhibitor activity assay. The significant reduction of the survival rate of the Angoumois grain moth (Sitotroga cerealella, Lepidoptera: Gelechiidae), reared on transgenic wheat seeds expressing the trypsin inhibitor BTI-CMe, compared to the untransformed control confirmed the potential of BTI-CMe for the increase of insect resistance. However, only early-instar larvae were inhibited in transgenic seeds and expression of BTI-CMe protein in transgenic leaves did not have a significant protective effect against leaf-feeding insects.
Similar content being viewed by others
References
Alfonso J, Ortego F, Sanchez-Monge R, Garcia-Casado G, Pujol I, Castanera P, Salcedo G: Wheat and barley inhibitors active towards _-amylase and trypsin-like activities from Spodoptera frugiperda. J Chem Ecol 23: 1729–1741 (1997).
Altpeter F, Vasil V, Srivastava V, Stoeger E, Vasil IK: Accelerated production of transgenic wheat plants. Plant Cell Rep 16: 12–17 (1996).
Altpeter F, Vasil V, Srivastava V, Vasil IK: Integration and expression of the high-molecular-weight glutenin subunit 1Ax1 gene into wheat. Nature Biotechnol 14: 1155–1159 (1996).
Boisen S: Comparative physico-chemical studies on purified trypsin inhibitors from endosperm of barley, rye and wheat. Z Lebensm Unters Forsch 176: 434–439 (1983).
Bown DP, Wilkinson HS, Gatehouse JA: Differentially regulated inhibitor-sensitive and insensitive protease genes from the phytophagus insect pest, Helicopverpa armigera, are members of complex multigene families. Insect Biochem Mol Biol 27: 625–638 (1997)
Brandon DL, Bates AH, Friedman M: ELISA analysis of soybean trypsin inhibitors in processed foods. In: Friedman M (ed) Nutrional and Toxicological Consequences of Food Processing, pp 321–337. Plenum Press, New York (1991).
Broadway RM: Are insects resistant to plant proteinase inhibitors? J Insect Physiol 41: 107–116 (1995).
Broadway RM: Dietary proteinase inhibitors alter complement of midgut proteases. Arch Insect Biochem Physiol 32: 39–53 (1996).
Carbonero P, Garcia-Olmedo F: Multigene family of trypsin/α-amylase inhibitors from cereals. In: Casey R, Shewry PR (eds) Cereal Proteins. Chapman and Hall, London (in press).
Christensen AH, Quail PH: Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgen Res 5: 213–218 (1996).
Christensen AH, Sharrock RA, Quail PH: Maize polyubiquitin genes: structure, thermal perturbation of expression and tanscript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18: 675–689 (1992).
Dennis MS, Lazarus RA: Kunitz domain inhibitors of tissue factor VIIa. I. Potent inhibitors selected from libraries by phage display. II Potent and specific inhibitors by competitive phage selection. J Biol Chem 269: 22129–22144 (1994).
Diaz I, Royo J, Carbonero P: The promoter of barley trypsininhibitor BTI-CMe, discriminates between wheat and barley endosperm protoplasts in transient expression assays. Plant Cell Rep 12: 698–701 (1993).
Garcia-Olmedo F, Salcedo G, Sanchez-Monge R, Gomez L, Royo J, Carbonero P: Plant proteinaceous inhibitors of proteinases and α-amylases. Oxf Surv Plant Mol Cell Biol 4: 275–334 (1987).
Gumbmann MR, Spangler WL, Dugan GM, Rackis JJ: Safety of trypsin inhibitors in the diet: effects on the rat pancreas of long-term feeding of soy flour and soy protein isolate. In: Friedman M (ed) Nutrional and Toxicological Significance of Enzyme Inhibitors in Foods, pp. 33–79. Plenum Press, New York (1986).
Hilder V A, Gatehouse AMR, Sheerman SE, Barker RF, Boulter D: A novel mechanism of insect resistance engineered into tobacco. Nature 330: 160–163 (1987).
Hinks CF, Olfert O, Westcott ND, Coxworth EM, Craig W: Preference and performance in grasshopper, Melanoplus sanguinipes (Orthoptera: Acrididae), feeding on kochia, oats, and wheat: Implications for population dynamics. J Econ Entomol 83: 1338–1343 (1990).
Hoffmann MP, Zalom FG, Wilson LT, Smilanick JM, Malyj LD, Kiser J, Hilder VA, Barnes WM: Field evaluation of transgenic tobacco containing genes encoding Bacillus thuringiensis endotoxin or cowpea trypsin inhibitor: efficacy against Helicoverpa zea (Lepidoptera: Noctuidae). J Econ Entomol 85, 2516–2522 (1992).
Jembere B, Obeng-Ofori D, Hassanali A: Products derived from leaves of Ocimum kilimandscharicum (Labiatae) as postharvest grain protectants against the infestation of three major stored product insect pests. Bull Entomol Res 85: 361–367 (1995).
Johnson R, Narvaez J, An G, Ryan C: Expression of proteinase inhibitors I and II in transgenic tobacco plants: Effects on nat-ural defence against Manduca sexta larvae. Proc Natl Acad Sci USA 86: 9871–9875 (1989).
Jongsma MA, Stiekema WJ, Bosch D: Combatting inhibitorinsensitive proteases of insect pests. Trends Biotechnol 14: 331–333 (1996)
Jongsma MA, Boulter C: The adaptation of insects to plant protease inhibitors. J Insect Physiol 43: 885–895 (1997)
Jongsma MA, Bakker P L, Peters J, Bosch D, Stiekema WJ: Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc Natl Acad Sci USA 92: 8041–8045 (1995).
Jouanin L, Bonande-Bottino M, Girard C, Morrot G, Giband M: Transgenic plants for insect resistance. Plant Sci 131: 1–11 (1998).
Kollipara KP, Singh L, Hymowitz H: Genetic variation of trypsin and chymotrypsin inhibitors in pigeonpea (Cajanus cajan L. Millsp.) and its wild relatives. Theor Appl Genet 88: 986–993 (1994).
Laemmli UK: Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 277: 680–685 (1970).
Lagrimini LM, Burkhart W, Moye M, Rothstein S: Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: molecular analysis and tissuespecific expression. Proc Natl Acad Sci USA 84: 7542–7546 (1987).
Lassner NW, Peterson P and Yoder JI: Simultaneous amplification of multiple DNA fragments by polymerase chain reaction in the analysis of transgenic plants and their progeny. Plant Mol Biol Rep 7: 116–128 (1989).
Liener IE: Trypsin inhibitors: concern for human nutrition or not? J Nutr 116: 920–923 (1986).
Liener I.E, Kakade ML: Proteinase inhibitors. In Liener IE (ed) Toxic Constituents of Plant Foodstuffs, pp. 7–71. Academic Press, New York (1980).
Mikola J, Suolinna E-M: Purification and properties of a trypsin inhibitor from barley. Eur J Biochem 9: 555–560 (1969).
Mitsunaga T: Isolation and characterization of trypsin inhibitors from wheat germ. J Nutr Sci Vitaminol (Tokyo) 25: 43–52 (1979).
Mossor G, Skupin J: Some biochemical properties of trypsin inhibitor type antinutrients derived from extracts of wheat grain, Beta variety. Nahrung 29: 491–500 (1985)
Odani S, Koide T, Ono T: The complete amino acid sequence of barley trypsin inhibitor. J Biol Chem 258: 7998–8003 (1983).
Roberts BL, Markland W, Ley AC, Kent RB, White DW, Guterman SK, Ladner RC: Directed evolution of a protein: Selection of potent neutrophil elastase inhibitors displayed on M13 fusion phage. Proc Natl Acad Sci USA 89: 2429–2433 (1992).
Rodriguez-Palenzuela P, Royo J, Gomez L, Sanchez-Monge R, Salcedo G, Molina-Cano JL, Garcia-Olmedo F, Carbonero P: The gene for trypsin inhibitor CMe is regulated in trans by the lys 3a locus in the endosperm of barley (Hordeum vulgare L.). Mol Gen Genet 219: 474–479 (1989).
Royo J, Diaz I, Rodriquez-Palenzuela P, Carbonero P: Isolation and promoter characterization of barley gene Itr1 encoding trypsin inhibitor BTI-CMe: differential activity in wild-type and mutant Lys3a endosperm. Plant Mol Biol 31: 1051–1059 (1996).
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
SAS Institute: SAS/STAT guide for personal computers. version 6.03. SAS Institute, Inc., Cary, NC (1990).
Spencer TM, Gordon-Kamm WJ, Daines RJ, Start WG, Lemaux PG: Bialaphos selection of stable transformants from maize cell culture. Theor Appl Genet 79: 625–631 (1990).
Urwin P, Atkinson H, Waller, D, McPherson M: Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida. Plant J 8: 121–131 (1995).
Vasil V, Srivastava V, Castillo AM, Fromm ME, Vasil IK: Rapid production of transgenic wheat plants by direct bombardment of cultured immature embryos. Bio/technology 11: 1553–1558 (1993).
Weaver DK, Throne JE: Life history data for Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) in farm-stored corn and the importance of suboptimal environmental conditions in insect population modelling for bulk commodities. In: Highley E, Wright EJ, Banks HJ, Champ BR (eds) Stored Product Protection, pp. 599–604. Proceedings 6th International Working Conference on Stored Product Protection. (17–23 April 1994, Canberra, Australia), vol. 1 (1994).
Wu Y, Llewellyn D, Mathews A, Dennis E: Adaptation of Helicoverpa armigera (Lepidoptera: Noctuidae) to a proteinase inhibitor expressed in transgenic tobacco. Mol Breed 3: 371–380 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Altpeter, F., Diaz, I., McAuslane, H. et al. Increased insect resistance in transgenic wheat stably expressing trypsin inhibitor CMe. Molecular Breeding 5, 53–63 (1999). https://doi.org/10.1023/A:1009659911798
Issue Date:
DOI: https://doi.org/10.1023/A:1009659911798