Abstract
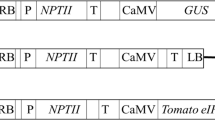
Potato leafroll poleovirus and the Colorado potato beetle (Leptinotarsa decemlineata (Say)) are major pests of potato in the USA. The US Department of Agriculture estimates that over 50% of annual insecticide use on potato is applied to control the Colorado potato beetle and aphids that transmit potato leafroll virus (PLRV). To address this issue, Russet Burbank potatoes have been genetically modified for a high level of resistance to infection and the resulting disease symptoms caused by PLRV and to feeding damage caused by the Colorado potato beetle. This resistance was achieved by the expression of the unmodified full-length replicase gene of PLRV and the cry3A insect control protein gene from Bacillus thuringiensis var. tenebrionis. Plant expression constructs containing various modifications of the PLRV replicase gene were produced during the development of this product. The genes in these constructs were a full-length unmodified replicase (open reading frame 2a/2b), an antisense orientation of the full-length cDNA, an open reading frame 1 translation of the full-length gene, and a gene truncation containing the 3′ sense coding portion of the replicase gene. Growth chamber experiments demonstrated that transformation of plants with the full-length and 3′ sense coding constructs substantially protected these potato plants from infection and disease symptoms caused by PLRV. The Russet Burbank potato expressing the full-length PLV replicase gene and the cry3A gene is a new potato product from NatureMark called NewLeaf Plus®.
Similar content being viewed by others
References
Almeida E.R.P., Gossele V., Muller C.G., Dockx J., Reynaerts A., Botterman J., krebbers E. and Timko M.P. 1989. Transgenic Expression of Two Marker genes under the control of an Arabidopsis rbcS promoter: sequences encoding the rubisco transit peptide increase expression level. Mol. Gen. Genet. 218: 78–86.
Anonymous 1995. Agricultural Chemical Usage: 1994 Field Crops Summary. USDA Natl Agric Stat Serv Ag Cl 106.
Almeida E.R.P., Gossele V., Muller C.G., Dockx J., Reynaerts A., Botterman J., Krebbers E., Timko M.P. 1989. Transgenic Expression of Two Marker genes under the control of an Arabidopsis rbcS promoter: sequences encoding the rubisco transit peptide increase expression level. Mol. Gen. Genet. 218: 78–86.
Audy P., Palukaitis P., Slack S. and Zaitlin M. 1994. Replicase-mediated resistance to potato virus Y in transgenic tobacco plants. Mol. Plant-Microbe Interact. 7: 15–22.
Austin G. 1994. Increasing the level of expression of foreign genes in plants using the non-translated leader sequence of a heat shock gene. U.S. Patent 5,362,865.
Bacon O.G., Burton V.E., McLean D.L., James R.H., Riley W.D., Baghott K.G. and Kinsey M.G. 1976. Control of green peach aphid and its effects on incidence of potato leafroll virus. J. Econ. Entomol. 69: 410–414.
Bahner I., Lamb J., Mayo M.A. and Hay R.T. 1990. Expression of the genome of potato leafroll virus: readthrough of the coat protein termination codon in vivo. J. Gen. Virol. 71: 2251–2256.
Barry G., Kishore G., Padgette S., Taylor M., Kolacz K., Weldon M., Re D., Eichholtz D., Fincher K. and Hallas L. 1992. Inhibitors of amino acid biosynthesis: Strategies for imparting glyphosate tolerance to crop plants. In: Singh B.K., Flores H.E. and Shannon J.C. (Eds.) Biosynthesis and Molecular Regulation of Amino Acids in Plants, American Society of Plant Physiologists, pp. 139–145.
Bevan M., Barnes W.M. and Chilton M. 1983. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucl. Acids Res. 11: 369–385.
Braun C.J. and Hemenway C.L. 1992. Expression of amino-terminal portions or full length viral replicase genes in transgenic plants confers resistance to potato virus X infection. Plant Cell 4: 735–744.
Carr J.P., Marsh L.E., Lonmonossoff G.P., Sekiya M.E. and Zaitlin M. 1992. Resistance to tobacco mosaic virus induced by the 54-kDa gene sequences requires expression of the 54-kDa protein. Mol. Plant-Microbe Interact. 5: 397–404.
Coruzzi G., Broglie R., Edwards C. and Chua N.-H. 1984. Tissuespecific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 3: 1671–1679.
Cuozzo M., O'Connel K.M., Kaniewski W.K., Fang R., Chua N. and Tumer N.E. 1988. Viral protection in transgenic tobacco plants expressing the cucumber mosaic virus coat protein or its antisense RNA. Bio/technology 4: 549–555.
Depicker A., Stachel S., Dhaese P., Zambryski P. and Goodman H.M. 1982. Nopaline synthase: transcript mapping and DNA sequence. J. Mol. Appl. Genet. 1: 561–573.
Ditta G., Stanfield S., Corbin D. and Helinski D.R.: 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77: 7347–7351.
Fauquet M.C. and Mayo M.A. 1999. Abbreviations for plant virus names-1999. Arch. Virol. 144: 1249–1262.
Fraley R.T., Rogers S.G., Horsch R.B., Sanders P.R., Flick J.S. and Adams S.P., Bittner M.L., Brand L.A., Fink C.L., Fry J.S., Galluppi G.R., Goldberg S.B., Hoffmann N.L., Woo S.C. 1983. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 80: 4803–4807.
Gal-On A., Wolf D., Wang Y., Faure J.-E., Pilowsky M. and Zelcer A. 1998. Transgenic resistance to cucumber mosaic virus in tomato: Blocking of long-distance movement of the virus in lines harboring a defective viral replicase gene. Phytopathology 88: 1101–1107.
Gowda S., Wu F.C. and Shepard R.J. 1989. Identification of promoter sequences for the major RNA transcripts of figwort mosaic and peanut chlorotic streak viruses (caulimovirus group). (Abstr) J. Cell. Biochem. Suppl. 13D: 301.
Habili N. and Symons R.H. 1989. Evolutionary relationship between luteoviruses and other RNA plant viruses based on sequence motifs in their putative RNA polymerases and nucleic acid helicases. Nucl. Acids. Res. 17: 9543–9555.
Hanifi A., Radcliffe E.B. and Ragsdale D. 1989. Spread and control of potato leafroll virus in Minnesota. J. Econ. Entomol. 82: 1201–1206.
Kamer G. and Agros P. 1984. Primary structural comparison of RNA-dependent polymerases from plant, animal, and bacterial viruses. Nucl. Acid Res. 12: 7269–7282.
Kaniewski W., Lawson C., Loveless J., Thomas P., Mowry T., Reed G., Mitsky T., Zalewski J. and Muskopf Y. 1995. Expression of potato leafroll virus (PLRV) replicase genes in Russet Burbank potatoes provide immunity to PLRV. In: Manka M. (Ed.), Environmental Biotic Factors in Integrated Plant Disease Control. Proceed. 3rd EFPP Conference. Polish Phytopathological Society, Poznan, Poland, pp. 289–292.
Kaniewski W. and Lawson C. 1998. Coat protein and replicasemediated resistance to plant viruses. In: Hadidi A., Khetarpar R.K. and Koganezwa H. (eds.), Plant Virus Disease Control. APS Press, St Paul, MN, pp. 65–78.
Klee H.J. and Rogers S.G. 1989. Plant gene vectors and genetic transformation: plant transformation systems based on the use of Agrobacterium tumefaciens. Cell Cult. Som. Cell Genet. Plants 6: 1–23.
Koev G., Mohan B.R., Dinesh-Kumar S.P., Torbert K.A., Somers D.A. and Miller W.A. 1998. Extreme reduction of disease in oats transformed with the 50 half of the barley yellow dwarf virus-PAV genome. Phytopathology 88: 1013–1019.
Koncz C. and Schell J. 1986. The promoter of T-L DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204: 383–396.
Longstaff M., Brigneti G., Boccard F., Chaoman S. and Baulcombe D. 1993. Extreme resistance to potato virus X infection in plants expressing a modified component of the putative viral replicase. EMBO J. 12: 379–386.
Martin R.R., Keese P.K., Young M.J., Waterhouse P.M. and GerlachW. L. 1990. Evolution andmolecular biology of luteoviruses. Annu. Rev. Phytopath. 28: 341–363.
McPherson S.A., Perlak F.J., Fuchs R.L., Marrone P.G., Lavrik P.B. and Fischhoff D.A. 1988. Characterization of the coleopteranspecific protein of Bacillus thuringiensis subsp. tenebrionis. Bio/technology 6: 61–66.
Miller W.A., Dinesh-Kumar S.P. and Paul C.P. 1995. Luteovirus gene expression. Crit. Rev. Plant Sci. 14: 179–211.
Mueller E., Gilbert J., Davenport G., Brigneti G. and Baulcombe D.C. 1995. Homology-dependent resistance: transgenic virus resistance in plants related to homology-dependent gene silencing. Plant J. 7: 1001–1013.
Newell C.A., Rozman R., Hinchee M.A., Lawson E.C., Haley L., Sanders P., Kaniewski W., Tumer N.E., Horsch R.B. and Fraley R.T. 1991. Agrobacterium mediated transformation of Solanum tuberosum L. cultivar Russet Burbank. Plant Cell Rep. 10: 30–34.
Palukaitis P. and Zaitlin M. 1997. Replicase-mediated resistance to plant virus disease. Adv. Virus Res. 48: 349–377.
Perlak F.J., Stone T.B., Muskopf Y.M., Petersen L.J., Parker G.B., McPherson S.A., Wyman J., Love S., Reed G., Biever D. and Fischhoff D.A. 1993. Genetically improved potatoes: Protection from damage by colorado potato beetles. Plant Mol. Biol. 22: 313–321.
Powell D.M. and Mandor W.T. 1976. Area control of the green peach aphid on peach and the reduction of potato leafroll virus. Am. Potato J. 53: 123–139.
Raschke E., Baumann G. and Schoffl F. 1988. Nucleotide sequence analysis of soybean small heat shock protein genes belonging to two different multigene families. J. Mol. Biol. 199: 549–557.
Richins R., Scholthof H. and Shepard R. 1987. Sequence of figwort mosaic virus DNA (caulimovirus group). Nucl. Acids Res. 15: 8451–8466.
Sambrook J., Fritsch E.F. and Maniatis T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor Laboratory, Plainview, NY.
Tacke E., Prufer D., Schmitz J. and Rohde W. 1991. The potato leafroll luteovirus 17K protein is a single-stranded nucleic acidbinding protein. J. Gen. Virol. 72: 2035–2038.
Thomas P.E., Zielinska L.M. and Smith D.R. 1978. Virus disease of potatoes in Washington, their costs and controls. Annu. Proc. Wash. Potato Conf. 17: 113–119.
Thomas P.E., Pike K.S. and Reed G.L. 1998. Sources, dissemination, and control of potato leafroll diseases. Annu. Proc. Wash. Potato Conf. 32: 141–146.
Thomas P.E., Reed G.L., Kaniewski W.K., Lawson E.C., Mowry T., Salais T. and Zalewski J. 1994. Elimination of potato leafroll disease and tuber net necrosis in transgenic Russet Burbank potatoes. Annu. Proc. Wash Potato Conf. 17: 59–64.
Thomas P.E., Kaniewski W.K. and Lawson E.C. 1997. Reduced field spread of potato leafroll virus in potatoes transformed with the potato leafroll virus coat protein gene. Plant Dis. 81: 1447–1453.
Thomas P.E., Lawson E.C., Zalewski J.C., Reed G.L. and Kaniewski W.K. 2001. Extreme field resistance to potato leafroll virus in Russet Burbank mediated by the viral replicase gene. Virus Res. 71(1-2): 49–62.
Tinland B. 1996. The Integration of T-DNA into plant genomes. Trends Plant Sci. 1: 178–184.
van der Wilk F., Huisman M.J., Cornelissen B.J.C., Huttinga H. and Goldbach R. 1989. Nucleotide sequence and organization of potato leafroll virus genomic RNA. FEBS Lett. 245: 51–56.
Wong E.Y., Hironaka C.M. and Fischhoff D. 1992. Arabidopsis thaliana small subunit leader and transit peptide enhances the expression of Bacillus thuringiensis proteins in transgenic plants. Plant Mol. Biol. 20: 81–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawson, E., Weiss, J., Thomas, P. et al. NewLeaf Plus® Russet Burbank potatoes: replicase-mediated resistance to potato leafroll virus. Molecular Breeding 7, 1–12 (2001). https://doi.org/10.1023/A:1009637325028
Issue Date:
DOI: https://doi.org/10.1023/A:1009637325028