Abstract
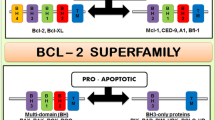
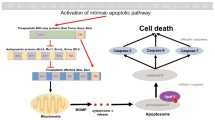
The Bcl-2 oncoprotein is a potent inhibitor of apoptosis induced by numerous physiological and pathological stimuli, and uncontrolled cell survival due to Bcl-2 overexpression has been shown to contribute to tumour formation and the development of autoimmune diseases. The multifunctional action of Bcl-2 is thought to prevent activation of the ced3/caspase-3 subfamily of ICE proteases, resulting in suppression of the death effector machinery. Since most conventional anti-cancer agents act by triggering this suicide pathway, overexpression of Bcl-2 in cancer cells has also been associated with drug resistance. The antisense approach to inhibition of gene expression relies on the binding of small synthetic oligodeoxynucleotides to a complementary base sequence on a target mRNA. As a consequence, expression of the corresponding gene is downregulated due to endonuclease-mediated hydrolysis of the mRNA strand, or to translational arrest arising from sterie hindrance by the RNA:DNA heterodimer. Since these mechanisms of action differ from those exerted by conventional anticancer agents, antisense oligodeoxynucleotides designed to specifically inhibit bcl-2 gene expression hold great promise as agents that could overcome clinical drug resistance, and improve the treatment outcome of many hitherto incurable cancer diseases.
Similar content being viewed by others
References
Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin 1994; 44: 7–26.
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456–1462.
Evan Gl, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992; 69: 119–128.
Marx J. Cell death studies yield cancer clues. Science 1993; 259: 760–761.
Steller H. Mechanisms and genes of cellular suicide. Science 1995; 267: 1445–1449.
Fisher DE. Apopcosis in cancer therapy: crossing the threshold. Cell 1994; 78: 539–542.
Tsujimoco Y, Croce CM. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA 1986; 83: 5214–5218.
Ben Ezra JM, Kornscein MJ, Grimes MM, Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol 1994; 145: 1036–1040.
Gee JM, Robertson JF, Ellis IO, et al. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer 1994; 59: 619–628.
Hanada M, Krajewski S, Tanaka S, e.t al. Regulation of Bcl-2 oncoprotein levels with differentiation of human neuroblastoma cells. Cancer Res 1993; 53: 4978–4986.
Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Srivascava S, Moul JW. Elevated levels of apoptosis regulator proteins p53 and bcl-2 are independent prognostic biomarkers in surgically treated clinically localized prostate cancer. J Urol 1996; 156: 1511–1516
Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124: 1–6.
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 1993; 53: 4701–4714.
Minn AJ, Velez P, Schendel SL, et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 1997; 385: 353–357.
Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA 1997; 94: 5113–5118.
Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature 1997; 3: 614–620.
Reed JC. Double identity for proteins of the bcl-2 family. Nature 1997; 387: 773–776.
Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene 1996; 12: 2251–2257.
Monney L, Otter I, Olivier R, et al. Bcl-2 overexpression blocks activation of the death protease CPP32/Yama/ apopain. Biochem Biophys Res Commun 1996; 221: 340–345.
Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem 1996; 271: 4573–4576.
Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 1993; 81: 151–157.
Dole M, Nunez G, Merchant AK, et al. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res 1994; 54: 3253–3259.
Ohmori T, Podack ER, Nishio K, et al. Apoptosis of lung cancer cells caused by some anti-cancer agents (MMC, CPT-11, ADM) is inhibited by bcl-2. Biochem Biophys Res Commun 1993; 192: 30–36.
Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993; 74: 609–619.
Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol 1993; 4: 327–332.
Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(l4;18) translocation. Cell 1986; 47: 19–28.
Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990; 348: 331–333.
Yunis JJ, Mayer MG, Arnesen MA, Aeppli DP, Oken MM, Frizzera G. bcl-2 and other genomic alterations in the prognosis of large-cell lymphoma. N Engl J Med 1989; 320: 1047–1054.
Campos L, Renault JP, Sabido O, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 1993; 81: 3091–3096.
Fontanini G, Vignati S, Bigini D, et al. Bcl-2 protein: a prognostic factor inversely correlated to p53 in nonsmall-cell lung cancer. Br J Cancer 1995; 71: 1003–1007.
Manne U, Myers RB, Moron C, et al. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer 1997; 74: 346–358.
Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem 1996; 271: 12695–12698.
Helene C, Toulme JJ. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta 1990; 1049: 99–125.
Milligan JF, Matteucci MD, Martin JC. Current concepts in antisense drug design. J Med Chem 1993; 36: 1923–1937.
Crooke ST. Therapeutic applications of oligonucleotides. Biotechnology NY 1992; 10: 882–886.
Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA 1978; 75: 280–284.
Kimura S, Maekawa T, Hirakawa K, Murakami A, Abe T. Alterations of c-myc expression by antisense oligodeoxynucleotides enhance the induction of apoptosis in HL-60 cells. Cancer Res 1995; 55: 1379–1384.
Monia BP, Johnston JF, Geiger T, Muller M, Fabbro D. Antitumor activity of a phosphororhioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nat Med 1996; 2: 668–675.
Mukhopadhyay T, Tainsky M, Cavender AC, Roth JA. Specific inhibition of K-ras expression and tumorigenicity of lung cancer cells by antisense RNA. Cancer Res 1991; 51: 1744–1748.
Skorski T, Nieborowska Skorska M, Wlodarski P, et al. Treatment of Philadelphia leukemia in severe combined immunodeficient mice by combination of cyclophosphamide and bcr/abl antisense oligodeoxynucleotides. J Natl Cancer Inst 1997; 89: 124–133.
Ziegler A, Luedke GH, Fabbro D, Altmann K-H, Stahel RA, Zangemeister-Wittke U. Induction of apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the bcl-2 coding sequence. J Natl Cancer lnst 1997; 89: 1027–1036.
Dean N, McKay R, Miraglia L, et al. Inhibition of Growth of Human tumor cell lines in nude mice by an antisense of oligonucleotide inhibitor of protein kinase C-alpha expression. Cancer Res 1996; 56: 3499–3507.
Stein CA, Subasinghe C, Shinozuka K, Cohen JS. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res 1988; 16: 3209–3221.
Wagner RW. The state of the art in antisense research. Nat Med 1995; 1: 1116–1118.
Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature 1994; 372: 333–335.
Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents - is the bullet really magical? Science 1993; 261: 1004–1012.
Gewirtz AM, Stein CA, Glazer PM. Facilitating oligonucleotide delivery: helping antisense deliver on its promise. Proc Natl Acad Sci USA 1996; 93: 3161–3163.
Bennett CF, Chiang MY, Chan H, Shoemaker JE, Mirabelli CK. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol 1992; 41: 1023–1033.
Matteucci MD, Wagner RW. In pursuit of antisense. Nature 1996; 384: 20–22.
Raynaud FI, Orr RM, Goddard PM, et al. Pharmacokinetics of G3139, a phosphorothioate oligodeoxynucleotide antisense to bcl-2, after intravenous administration or continuous subcutaneous infusion to mice. J Pharmacol Exp Ther 1997; 281: 420–427.
Pisetsky DS. Immunologic consequences of nucleic acid therapy. Antiseme Res Dev 1995; 5: 219–225.
Galbraith WM, Hobson WC, Giclas PC, Schechter PJ, Agrawal S. Complement activation and hemodynamic changes following intravenous administration of phosphorothioate oligonucleocides in the monkey. Antisense Res Dev 1994; 4: 201–6X.
Monia BP, Sasmor H, Johnston JF, et al. Sequence-specific antitumor activity of a phosphorothioate oligodeoxyribonucleotide targeted to human C-raf kinase supports an antisense mechanism of action in vivo. Proc Natl Acad Sci USA 1996; 93: 15481–15484.
Guvakova MA, Yakubov LA, Viodavsky I, Tonkinson JL, Stein CA. Phosphorochioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem 1995; 270: 2620–2627.
Berchem GJ, Bosseler M, Sugars LY, Voeller HJ, Zeitlin S, Gelmann EP. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancel-Res 1995; 55: 735–738.
Cotter FE, Johnson P, Hall P, et al. Antisense oligonucleotides suppress B-cell lymphoma growth in a SCID-hu mouse model. Oncogene 1994; 9: 3049–3055.
Reed JC, Stein C, Subasinghe C, et al. Antisense-mediated inhibition of BCL-2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res 1990; 50: 6565–6570.
Kitada S, Miyashita T, Tanaka S, Reed JC. Investigations of antisense oligonucleotides targeted against bcl-2 RNAs. Antisense Res Dev 1993; 3: 157–169.
Kitada S, Takayama S, De Riel K, Tanaka S, Reed JC. Reversal of chernoresistance of lymphoma cells by antisense-mediated reduction of bcl-2 gene expression. Antisense Res Dev 1994; 4: 71–79.
Campos L, Sabido O, Rouault JP, Guyotat D. Effects of BCL-2 antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood 1994; 84: 595–600.
Bacon TA, Wickstrom E. Walking along human c-myc mRNA with antisense oligodeoxynucleotides: maximum efficacy at the 5' cap region. Oncogene Res 1991; 6: 13–19.
Robinson LA, Smith LJ, Fontaine MP, Kay HD, Mountjoy CP, Pirruccello SJ. c-myc antisense oligodeoxyribonucleotides inhibit proliferation of non-small cell lung cancer. Ann Thorac Surg 1995; 60: 1583–1591.
Cotter FE, Corbo M, Raynaud F, et al. Bcl-2 antisense therapy in lymphoma: in vitro and in vivo mechanisms, efficacy, pharmacokinetic and toxicity studies. Ann Oncol 1996; 7: 100.
Webb A, Cunningham D, Cotter F, et al. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet 1997; 349: 1137–1141.
Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res 1995; 55: 3902–3907.
Morelli S, Delia D, Capaccioli S, et al. The ancisense bcl-2-IgH transcript is an optimal target for synthetic oligonucleotides. Proc Natl Acad Sci USA 1997; 94: 8150–8155.
Simonian PL, Grillot DA, Nunez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 1997; 90: 1208–1216.
Matsuzaki Y, Nakayama K, Tomita T, Isoda M, Loh DY, Nakauchi H. Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood 1997; 89: 853–862.
Froesch BA, Luedke GH, Ziegler A, Stahel RA, Zangemeister-Wittke U. The synergistic cytotoxic effect of a doxorubicin immunoconjugate and bcl-2 antisense oligonucleotides on non-resistant and drug resistant small cell lung cancer cell lines. Tumor Targeting 1996; 2: 265–276.
Matzura O, Wennborg A. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows. Computer Applications in Biosciences (CABIOS) 1996; 12: 247–249.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zangemeister-Wittke, U., Ziegler, A. Bcl-2 Antisense Therapy for Cancer: The Art of Persuading Tumour Cells to Commit Suicide. Apoptosis 3, 67–74 (1998). https://doi.org/10.1023/A:1009636722713
Issue Date:
DOI: https://doi.org/10.1023/A:1009636722713