Abstract
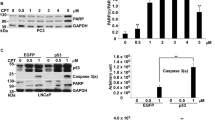
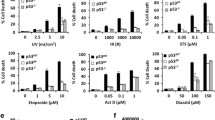
The p53 tumour suppressor is stabilised following exposure to genotoxic agents, such as γ-radiation. Cell responses to p53 stabilisation include induction of apoptosis and/or cell cycle arrest. Several studies have suggested that γ-radiation stabilises p53 by blocking ubiquitin mediated proteolysis. Here we have compared the biological activities of p53 stabilized following exposure to γ-radiation or treatment with the proteosome inhibitor N-acetyl-leucinyl-leucinyl-norleucinal (ALLN) in MCF7 cells with wild type p53. Stabilisation of p53 by ALLN was reversible and was not blocked by caffeine. Although ALLN was a more effective p53 stabilising agent than γ-radiation, ALLN was not as effective at inducing cell cycle arrest/apoptosis as γ-radiation. Although p53 stabilised by ALLN and γ-radiation were both able to bind DNA and activate transcription, ALLN did not increase expression of BAX, which is involved in p53-induced apoptosis. Therefore, p53 stabilised by different agents is not always biologically active to the same extent and additional alterations triggered by γ-radiation may enable p53 to activate a subset of critical target genes, such as BAX, which are required for p53 responses.
Similar content being viewed by others
References
Ko LJ, Prives C. p53: Puzzle and paradigm. Genes and Develop 1996; 10: 1054–1072.
El-Deiry W, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumour suppression. Cell 1993; 75: 817–825.
Miyashita T, Reed JC. Tumour suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80: 293–299.
Hollstein M, Sidransky D, Vogelstein B, Harris C. p53 mutations in human cancer. Science 1991; 253: 49–53.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–331.
Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res 1996; 56: 2649–2654.
Kubbutat MHG, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol 1997; 17: 460–468.
Pariat M, Carillo S, Molinari M, et al. Proteolysis by calpains: a possible contribution to degradation of p53. Mol Cell Biol 1997; 17: 2806–2815.
Fredersdorf S, Milne AW, Hall PA, Lu X. Characterisation of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol 1996; 148: 825–835.
Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, al. e, Lu X. High level expression of p27kip1 and cyclin D1 in some proliferating breast tumour cells: inverse correlation with the degree of malignancy in human breast and colorectal cancers. Proc Nat Acad Sci USA 1997; 94: 6380–6385.
Lu X, Lane DP. Differential induction of transcriptionally active p53 following UV or ionising radiation: defects in chromosome instability syndromes? Cell 1993; 75: 765–778.
O'Connor DJ, Lam EW-F, Griffin S, et al. Physical and functional interactions between p53 and cell cycle co-operating transcription factors, E2F1 and DP1. EMBO 1995; 14: 6184–6192.
Hsieh J-K, Fredersdorf S, Kauzarides T, Martin K, Lu X. E2F1 induced apoptosis requires DNA binding but not transcriptional activity and is inhibited by the retinoblastoma protein through direct interaction. Genes & Develop 1997; 11: 1840–1852.
Hupp TR, Meek DW, Midgley CA, Lane DP. Regulation of the specific DNA binding function of p53. Cell 1992; 71: 875–886.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991; 51: 6304–6311.
Maki CG, Howley PM. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol 1997; 17: 355–363.
Lu X, Burbidge SA, Griffin S, Smith HM. Discordance between accumulated p53 protein level and its transcriptional activity in response to UV radiation. Oncogene 1996; 13: 413–418.
Dietrich C, Bartsch T, Schanz F, Oesch F, Wieser RJ. p53-dependent cell cycle arrest induced by N-acetyl-L-leucinyl-L-leuinyl-L-norleucinal in platelet-derived growth factor-stimulated human fiboblasts. Proc Natl Acad Sci USA 1996; 93: 10815–10819.
Midgley CM, Owens B, Briscoe CV, Thomas DB, Lane DP, Hall PA. Coupling between g irradiation, p53 induction, and the apoptotic response depends upon cell type in vivo. J Cell Sci 1995; 108: 1843–1848.
Linke SP, Clarkin KC, Leonardo AD, Tsou A, Wahl GM. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depeletion in the absence of detectable DNA damage. Genes & Develop 1996; 10: 934–947.
Ludwig RT, Bates S, Vousden KH. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol Cell Biol 1996; 16: 4952–4960.
Friedlander P, Haupt Y, Prives C, Oren M. A mutant p53 that discriminate between p53-responsive genes cannot induce apoptosis. Mol Cell Biol 1996; 16: 4961–4971.
Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by mdm2. Cell 1997; 91: 325–334.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fallis, L.H., Richards, E., O'Connor, D.J. et al. The biological response of MCF7 breast cancer cells to proteosome inhibition or γ-radiation is unrelated to the level of p53 induction. Apoptosis 4, 99–107 (1999). https://doi.org/10.1023/A:1009614726059
Issue Date:
DOI: https://doi.org/10.1023/A:1009614726059