Abstract
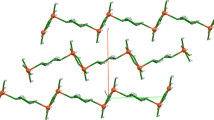
Previous studies of macrocycles having alkyl sidearms terminated in adenine or thymine have been focused on intermolecular association. Electrospray mass spectrometric analyses suggested that sodium complexation could be a major alternative process in the presence of the appropriate cation. Sodium cation complexation by N,N′-bis(3-(1-thyminyl)propyl)-4,13-diaza-18-crown-6 has been confirmed by X-ray crystallographic analysis. A sodium cation is bound in the center of the macroring. The four ether oxygens and two thyminyl carbonyl donors have the shortest contacts with the two, macroring nitrogen atoms being somewhat more remote. Neither the iodide counterion nor an acetone of solvation appears to interact with the sodium cation. Crystal Data: Triclinic, \(P_{\bar 1} \) , a = 11.9371(12), b = 12.1588(13), c = 13.7885(14) Å, α = 78.083(2)°, β = 83.213(2)°, γ = 78.997(2)°, and Dcalc = 1.392 g cm−3, Z = 2.
Similar content being viewed by others
References
(a) Aoyama, Y.; Onishi, H.; Tanaka, Y. Tetrahedron Lett. 1990, 1177. (b) Hisatome, M.; Maruyama, N.; Furutera, T.; Ishikawa, T.; Yamakawa, K. Chem. Lett. 1990, 2251. (c) Nowick; J.S.; Chen, J.S.; Noronha, G. J. Am. Chem. Soc. 1993, 115, 7636.
(a) Askew, B.; Ballester, P.; Buhr, C.; Jeong, K.S.; Jones, S.; Parris, K.; Williams, K.; Rebek Jr., J. J. Am. Chem. Soc. 1989, 111, 1082 and 1090. (b) Tecilla, P.; Dixon, R.P.; Slobodkin; G.; Alavi, D.S.; Waldeck, D.H.; Hamilton, A.D. J. Am.Chem. Soc. 1990, 112, 9408. (c) Zimmerman, S.C.Wu,W.; Zeng, Z. J. Am. Chem. Soc. 1991, 113, 196. (d) Cochram, J.E.; Parrott, T.J.; Whitlock, B.J.; Whitlock, H.W. J. Am. Chem. Soc. 1992, 114, 2269. (e) Diederich, F.; Smithrud, D.B.; Sanford, E.M.;Wyman, T.B.; Ferguson, S.B.; Carcanague, D.R.; Chao, I.; Houk, K.N. Acta Chim. Scand. 1992, 46, 205.
Furuta, H.; Magda, D.; Sessler, J.L. J. Am. Chem. Soc. 1991, 113, 978 and 4706.
Nagai, K.; Hayakawa, K.; Ukai, S.; Kanematsu, K. J. Org. Chem. 1986, 51, 3931.
(a) Van Staveren, C.J.; Van Eerden, J.; VanVeggel, J.; Harkema, F.C.; Reinhoudt, D.N. J. Am. Chem. Soc. 1988, 110, 4994. (b) Rebek J. Jr., Acc. Chem. Res. 1990, 23, 399. (c) Galan, A.; De Mendoza, J.; Toiron, C.; Bruix, M.; Deslongchamps, G.; Rebek J. Jr., J. Am. Chem. Soc. 1991, 113, 9424. (d) Nowick, J.S.; Feng, Q.; Tjivikua, T; Ballester, P.; Rebek Jr., J. J. Am. Chem. Soc. 1991, 113, 8831. (e) Inouye, M.; Konishi, T; Isagawa, T. J. Am. Chem. Soc. 1993, 115, 8091.
(a) Schall, O.F.; Gokel, G.W. J. Chem. Soc. Chem. Commun. 1992, 748-750. (b) Lynn, B.C.; Tsesarskaja, M.; Schall, O.F.; Hernandez, J.C.;Watanabe, S.; Kaifer, A.; Atwood, J.L.; Gokel, G.W. Supramol. Chem. 1993, 1, 253–260. (c) Schall,O.F.; Gokel, G.W. J. Am. Chem. Soc. 1994, 116, 6089–6100. (d) Schall, O.F.; Gokel, G.W. J. Org. Chem. 1996, 61, 1449–1458. (e) Wang, K.; Schall, O.F.; Gokel, G.W. Supramol. Chem. 1996, 7, 85–90.
Blessing, R.H. Acta Cryst. 1995, A51, 33-8.
Sheldrick, G.M. SHELX97, Program for Crystal Structure Refinement; University of Göttingen: Germany, 1997.
Barbour, L.J. X-Seed; University of Missouri-Columbia: USA, 1999 (http://www.lbarbour.com/xseed/).
Internet site to access Pov-Ray: http://www.povray.org.
(a) Gatto, V.J.; Gokel, G.W. J. Am. Chem. Soc. 1984, 106, 8240-8244. (b) Gatto, V.J.; Arnold, K.A.; Viscariello, A.M.; Miller, S.R.; Gokel, G.W. Tetrahedron Lett. 1986, 327–330.
Arnold, K.A.; Echegoyen, L.; Gokel, G.W. J. Am. Chem. Soc. 1987, 109, 3713-3715.
Arnold, K.A.; Viscariello, A.M.; Kim, M.; Gandour, R.D.; Fronczek, F.R.; Gokel, G.W. Tetrahedron Lett. 1988, 3025-3028.
Kim, M.; Gokel, G.W.; J. C. S. Chem. Commun. 1987, 1686-1688.
(a) De Wall, S.L.; Meadows, E.S.; Barbour, L.J.; Gokel, G.W.; J. Am. Chem. Soc. 1999, 121, 5613-5614. (b) De Wall, S.L.; Barbour, L.J.; Gokel, G.W. J. Am. Chem. Soc. 1999, 121, 8405–8406. (c) De Wall, S.L.; Meadows, E.S.; Barbour, L.J.; Gokel, G.W. Proc. Natl. Acad. Sci. USA 2000, 97, 6271–6276.
Wang, K.; Schall, O.F.; Gokel, G.W. Supramol. Chem. 1996, 7, 85-90.
Weintraub, S.T.; Pinckard, R.N.; Hail, M. Rapid Commun. Mass Spectrom. 1991, 5, 309.
Gandour, R.D.; Fronczek, F.R.; Gatto, V.J.; Minganti, C.; Schultz, R.A.; White, B.D.; Arnold, K.A.; Mazzocchi, D.; Miller, S.R.; Gokel, G.W. J. Am. Chem. Soc. 1986, 108, 4078-4088.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wall, S.L.D., xbour, L.J., Schall, O.F. et al. Sodium cation complexation in a macrocycle containing thymines as sidearm donor groups. Journal of Chemical Crystallography 30, 227–231 (2000). https://doi.org/10.1023/A:1009534904760
Issue Date:
DOI: https://doi.org/10.1023/A:1009534904760