Abstract
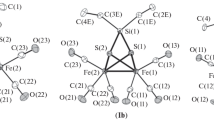
The tetrahedrane cluster, FeCo2(CO)9(μ3-S), reacts with the redox-active ligand, 4,5-bis(diphenylphosphino)-4-cyclopenten-1,3-dione (bpcd), to give the disubstituted cluster, FeCo2(CO)7(bpcd)(μ3-S), as the sole product. This diphosphine-substituted cluster contains a cobalt-bound, chelating bpcd ligand. Both IR and 31P NMR spectroscopies have been employed in the solution characterization of FeCo2(CO)7(bpcd)(μ3-S), and the solid-state structure has been unequivocally established by X-ray diffraction analysis. FeCo2(CO)7(bpcd)(μ3-S) crystallizes in the monoclinic space group C2/c, a = 34.494(3) Å, b = 11.4194(9) Å, c = 18.634(2) Å, β = 98.103(7)°, V = 7266.7(9) Å3, Z = 8, and dcalc = 1.584 g/cm3. Cyclic voltammetric studies on FeCo2(CO)7(bpcd)(μ3-S) reveal the presence of two quasireversible redox responses assigned to the 0/1− and 1−/2− redox couples. The orbital composition of these redox couples has been examined by carrying out extended Hückel MO calculations on the model complex FeCo2(CO)7(H4-bpcd)(μ3-S), with the results being compared to related cluster compounds.
Similar content being viewed by others
References
Braunstein, P.; Rosé, J. In Catalysis by Di-and Polynuclear Metal Cluster Complexes, Adams, R.D.; Cotton, F.A., Eds.; Wiley-VCH: New York, 1998; Chap. 13.
Gladfelter, W.L.; Geoffroy, G.L. Adv. Organomet. Chem. 1980, 18, 207.
Don, M.J.; Richmond, M.G.; Watson, W.H.; Krawiec, M.; Kashyap, R.P. J. Organomet. Chem. 1991, 418, 231. (b) Watson, W.H.; Nagl, A.; Hwang, S.; Richmond, M.G. J. Organomet. Chem. 1993, 445, 163. (c) Yang, K.; Bott, S.G.; Richmond, M.G. J. Organomet. Chem. 1993, 454, 273.
Yang, K.; Smith, J.M.; Bott, S.G.; Richmond, M.G. Organometallics 1993, 12, 4779. (b) Shen, H.; Bott, S.G.; Richmond, M.G. Inorg. Chim. Acta 1996, 250, 195.
Vahrenkamp, H. Inorg. Synth. 1989, 26, 352.
Fenske, D.; Becher, H.J.; Chem. Ber. 1975, 108, 2115.
Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Wiley: New York, 1980.
Hoffmann, R.; Lipscomb, W.N. J. Chem. Phys. 1962, 36, 2179. (b) Hoffmann, R. J. Chem. Phys. 1963, 39, 1397.
Mealli, C.; Proserpio, D.M. J. Chem. Ed. 1990, 67, 399.
Weast, R.C. (Ed.) Handbook of Chemistry and Physics, CRC Press: Cleveland, OH, 56th ed., 1975.
Koelle, U. J. Organomet. Chem. 1977, 133, 53. (b) Albers, M.O.; Coville, N.J. Coord. Chem. Rev. 1984, 53, 227.
Shen, H.; Bott, S.G.; Richmond, M.G. Organometallics 1995, 14, 4625.
We have only shown the two most likely possibilities of FeCo2 (CO)7(bpcd)(µ 3-S) that would display equivalent 31P centers.
Richmond, M.G.; Kochi, J.K. Inorg. Chem. 1986, 25, 1334.
Aime, S.; Milone, L.; Rossetti, R.; Stanghellini, P.L. Inorg. Chim. Acta 1977, 25, 103.
Colbran, S.B.; Robinson, B.; Simpson, J. Acta Crystallogr. 1986, C42, 972. (b) Ahlgre´ n, M.; Pakkanen, T.T.; Tahvanainen, I. J. Organomet. Chem. 1987, 323, 91. (c) Richter, F.; Vahrenkamp, H. Angew. Chem. Int. Ed. Eng. 1978, 17, 444. (d) Steveson, D.L.; Wei, C.H.; Dahl, L.F. J. Am. Chem. Soc. 1971, 93, 6027.
Richter, F.; Vahrenkamp, H. Chem. Ber. 1982, 115, 3243. (b) Richter, F.; Roland, E.; Vahrenkamp, H. Chem. Ber. 1984, 117, 2429. (c) Richter, F.; Vahrenkamp, H.; Organometallics 1982, 1, 756.
Don, M.-J.; Richmond, M.G.; Watson, W.H.; Kashyap, R.P. Acta Crystallgr. 1991, C47, 20. (b) Downard, A.J.; Robinson, B.H.; Simpson, J. Organometallics 1986, 5, 1122.
Honrath, U.; Vahrenkamp, H. Z. Naturforsch. 1984, 39B, 545.
Peake, B.M.; Rieger, P.H.; Robinson, B.H.; Simpson, J. Inorg. Chem. 1981, 20, 2540. (b) Bond, A.H.; Peake, B.M.; Robinson, B.H.; Simpson, J. Inorg. Chem. 1977, 16, 410. (c) Peake, B.M.;Robinson, B.H.; Simpson, J.; Watson, D.J. Inorg. Chem. 1977, 16, 405. (d) Peake, B.M.; Rieger, P.H.; Robinson, B.H.; Simpson, J. Inorg. Chem. 1979, 18, 1000.
Schilling, B.E.R.; Hoffmann, R. J. Am. Chem. Soc. 1979, 101, 3456. (b) Chesky, P.T.; Hall, M.B. Inorg. Chem. 1981, 20, 4419.
Schut, D.M.; Keana, K.J.; Tyler, D.R.; Rieger, P.H. J. Am. Chem. Soc., 1995, 117, 8939. (b) Meyer, R.; Schut, D.M.; Keana, K.J.; Tyler, D.R. Inorg. Chim. Acta 1995, 240, 405.
Albright, T.A.; Burdett, J.K.; Whangbo, M.H. Orbital Interactions in Chemistry, Wiley: New York, 1985.
Unpublished results.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bott, S.G., Wang, J.C., Shen, H. et al. Chelation of a diphosphine ligand at FeCo2(CO)9(μ3-S): Spectroscopic and X-ray diffraction data for FeCo2(CO)7(bpcd)(μ3-S). Journal of Chemical Crystallography 29, 391–397 (1999). https://doi.org/10.1023/A:1009502708303
Issue Date:
DOI: https://doi.org/10.1023/A:1009502708303