Abstract
Purpose: The objective was to study whether apoptosis occurs in human embryogenesis.
Methods: Human viable, arrested, and nonviable embryos and immature, and nonfertilized oocytes donated by our patients were used to detect apoptosis by Tunel labeling, annexin staining, and single-cell reverse transcriptase–polymerase chain reaction (RT-PCR).
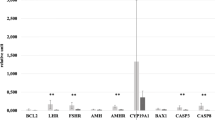
Results: DNA fragmentation and phosphotidylserine translocation, the two markers for apoptosis, were detected frequently in fragmented human embryos derived from in vitro fertilization–embryo transfer (IVF-ET). Using RT-PCR, apoptotic genes also were detected in these embryos. The frequencies of gene expression in viable embryos, arrested embryos, nonviable embryos, immature oocytes, and nonfertilized oocytes were: 7/8, 5/5, 5/6, 0/6, 0/3, for Bax; 8/8, 5/5, 7/7, 0/4, 0/5 for Fas; 2/8, 0/2, 0/3, 0/5, 0/3 for BCL-2; 0/8, 1/3, 0/2, 0/3, 0/2 for Fas-ligand; and 8/8, 17/17, 21/21, 24/24, 15/15 for actin, respectively.
Conclusions: Our preliminary data did not show a significant difference in the expression frequency of all studied genes between viable embryos and nonviable or arrested embryos. However, the expression of Bax and Fas was noticeably higher in nonviable embryos than in viable embryos as judged by the intensities of amplicons visualized after ethidium bromide staining. In addition, BCL-2 was only detected in viable embryos. Whether embryos quality is related to the regulation of BCL-2, Bax, and Fas expressions requires further study.
Similar content being viewed by others
REFERENCES
Glucksmann A: Cell deaths in normal vertebrate ontogeny. Biol Rev 1951;26:59-86
Kerr JFR, Wyllie AH, Currie AR: Apoptosis: A basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer 1972;26:239-257
Kerr JFR, Winterford CM, Harmon BV: Morphological criteria for identifying apoptosis. In Cell Biology: A Laboratory Handbook, JC Celis (ed). San Diego, CA, Academic Press, 1994, pp 319-329
Joensuu H, Pylkkanen L, Toikanen S: BCL-2 protein expression and long-term survival in breast cancer. Am J Pathol 1994;145:1191-1198
Akbar AN, Salmon M, Janossy G: Role of BCL-2 and apoptosis in viral infections. Int Arch Allergy Immunol 1994;105(4):359-362
Fisher GH, Rosenberg FJ, Strauss SE, Dale JK, Middleton LA, Lin AY, Strober W, Leonardo MJ, Puck JM: Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995;81(6):935-946
Lombardi VR, Miguel-Hidalgo JJ, Mancino G, Maneiro E, Poccia F, Placido R, Lagares R, Cacabelos R: Apoptosis during HIV infection and in nueurodegenerative disorders. Methods Find Exp Clin Pharmacol 1996;18(8):539-555
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, Van Schie RC, La Face DM, Green DR: Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995;182: 1545-1556
Wyllie AH: Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980;284:555-556
Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, Nakamura H, Ogita S, Sato E, Inoue M: DNAfragmentation of oocytes in aged mice. Hum Reprod 1996;11(7):1480-1483
Perez G, Tilly J: Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum Reprod 1997;12:2781-2783
Van Blerkom J, Davis P: DNA strand breaks and phosphatidylserine redistribution in newly ovulated and cultured mouse and human oocytes: occurrence and relationship to apoptosis. Hum Reprod 1998;13(5):1317-1324
Jurisicova A, Varmuza S, Casper RF: Programmed cell death and human embryo fragmentation. Mol Hum Reprod 1996;2:101-106
Korsmeyer SJ: BCL-2 initiates a new category of oncogenes: Regulators of cell death. Blood 1992;80:879-886
Hockenbery D: BCL-2 in cancer, development and apoptosis. J Cell Sci 1994;18:51-55
Oltvali Z, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993;74:609-619
Korsmeyer S, Shutter J, Veis D, et al.: BCL-2/Bax: A rheostat that regulates an antioxidant pathway and cell death. Semin Cancer Biol 1993;4:327-332
Oehm A, Behrmann I, Falk W, et al.: Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily; sequence identity with the Fas antigen. J Biol Chem 1992;267:10709-10715
Watanabe-Fukunaga R, Brannan CI, Itoh N, et al.: The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol 1992;148:1274-1279
Nagata S, Goldstein P: The Fas death factor. Science 1995;267(5203):1449-1456
Nagata S: Fas-mediated apoptosis. Adv Exp Med Biol 1996;406:119-124
Suda T, Takahashi T, Goldstein P, et al.: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis family. Cell, 1993;75:1169-1178
Tilly JL: Apoptosis and ovarian function. Rev Reprod 1996;1:162-172
Tilly JL, Tilly KI, Perez GI: The genes of cell death and cellular susceptibility to apoptosis in the ovary: A hypothesis. Cell Death Different 1997;4:180-187
Knudson CM, Tung KSK, Tourtellotte WG, et al.: Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995;270:96-99
Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL: Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 1999;21:200-203
Perez GI, Knudson CM, Leykin L, et al.: Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nature Med 1997;3:1228-1232
Knudson CM, Tung SKT, Tourtellotte WG, Brown GAJ, Korsmeyer SJ: Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96-99, 1995
Ratts VS, Flaws JA, Kolp R, et al.: Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 1995;136:3665-3668
Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL: Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol 1999;13(6):841-850
Guo MW, Mori E, Xu JP: Identification of Fas antigen associated with apoptotic cell death in the murine ovary. Biochem Biophys Res Commun 1994;203:1438-1446
Hakuno N, Koji T, Yano T, et al.: Fas/APO-1CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 1996;137:1938-1948
Kondo H, Maruo T, Peng X, et al.: Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicle regression and atresia. J Clin Endocrinol Metab 1996;81:2702-2710
Jurisicova A, Latham KE, Casper RF, Varmuza SL: Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev 1998;51(3):243-253
Brenner CA, Exley GE, Alikani M, McElhinny AS, Adler RR, Cohen J, Warner CM: Expression of Bax mRNA associated with apoptosis in human oocytes and embryos. (Abstract 1997:OC-19-163). In 10th World Congress on IVF and Assisted Reproduction. Vancouver, B.C. May 24-28, 1997
Warner CM, Cao W, Exley EG, McElhinney AS, Alikani M, Cohen J, Scott RT, Brenner CA: Genetic regulation of egg and embryo survival. Hum Reprod 1998;13(Suppl. 3):178-196
Antczak M, Van Blerkom J: Temporal and spatial aspects of fragmentation in early human embryos: Possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod 1999;14:429-447
Erenus M, Zouves C, Rajamahendram P, et al.: The effect of embryo quality on subsequent pregnancy rates after in vitro fertilization. Fertil Steril 1991;56:707-710
Jurisicova A, Rogers I, Fasciani A, Casper RF, Varmuza S: Effect of maternal age and conditions of fertilization on programmed cell death during murine preimplantation embryo development. Hum Reprod 1998;4(2):139-145
Liu HC, He ZY, Tang YX, Mele CA, Veeck LL, Davis OK, Rosenwaks Z: Simultaneous detection of multiple gene expression in mouse and human individual preimplantation embryos. Fertil Steril 1997;67(4):733-741
Grifo JA, Tang YX, Munne S, Alikani M, Cohen J, Rosenwaks Z: Heathy deliveries from biopsied human embryos. Hum Reprod 1994;9(5):912-916
Williams JR, Little JB, Shipley WU: Association of mammalian cell death with specific endonucleolytic degradation of DNA. Nature 1974;252:754-756
Welsh AO: Uterine cell death during implantation and early plantation. Microsc Res Tech 1993;25:113-245
El-Shershaby AM, Hinchliffe JR: Cell redundancy in the zona intact preimplantation mouse blastocyst: A light and electron microscope study of dead cells and their fate. J Embryol Exp Morphol 1993;25(3):223-245
Enders AD, Schlafke S: Differentiation of the blastocyst of the rhesus monkey. Am J Anat 1981;162:1-21
Mohr LR, Trounson AO: Comparative ultrastructure of hatched human mouse and bovine blastocysts. J Reprod Fertil 1982;66:499-504
Hardy K, Handyside AH, Winston RML: The human blastocyst: Cell number, death and allocation during late preimplantation development in vitro. Development 1989;107: 597-604
Biggers JD, Whitten WK, Whittingham DG: The culture of mouse embryos in vitro. In Methods in Mammalian Embryology. JC Daniel (ed). San Francisco, Freeman, 1971, pp 86-116
Weil M, Jacobson MD, Coles HSR, Davies TJ, Gardner RL, Raff KD, Raff MC: Constitutive expression of the machinery for programmed cell death. J Cell Biol 1996;133:1053-1059
Kawahara A, Kobayashi T, Nagata S: Inhibition of Fasinduced apoptosis by Bcl-2. Oncogene 1998;17(20):2549-2554
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW: Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991;51:6304-6311
Yang E, Zha J, Jackel J, Boise LH, Thompson CB, Korsmeyer SJ: Bad, a heterodimeric partner for Bcl-xl and Bcl-2 displaces Bax and promotes cell death. Cell 1995;80:285-291
Plachot M, Mandelbaum J: Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull 1990;46: 675-694
Cohen J, Alikani M, Liu HC, Rosenwaks Z: Rescue of human embryos by micromanipulation. Baillieres Clin Obstet Gynaecol 1994;8(1):95-116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, HC., He, ZY., Mele, C.A. et al. Expression of Apoptosis-Related Genes in Human Oocytes and Embryos. J Assist Reprod Genet 17, 521–533 (2000). https://doi.org/10.1023/A:1009497925862
Issue Date:
DOI: https://doi.org/10.1023/A:1009497925862