Abstract
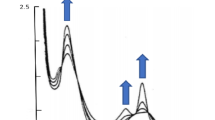
The iron-bleomycin complex has been shown to catalyze the oxidation of p-phenylenediamine to a stable, purple coloured oxidation product, characterized by an absorption maximum around 520 nm. Molecular oxygen is used for reoxidizing Fe(II)-bleomycin after reduction by p-phenylenediamine. An apparent Michaelis constant of 5.2 mM and a catalytic constant of 17.2 min−1 were obtained from kinetic studies. ATP, ADP and orthophosphate inhibited the catalytic oxidation of p-phenylenediamine, while AMP was without effect. It is proposed that p-phenylenediamine may be used as ‘substrate’ in kinetic studies involving the ‘oxidase’ activity of iron-bleomycin.
Similar content being viewed by others
References
Buettner GR, Moseley PL. 1992 Ascorbate both activates and inactivates bleomycin by free radical generation. Biochemistry 31, 9784-9788.
Burger RM, Peisach J, Blumberg WE, Horwitz SB. 1979 Ironbleomycin interactions with oxygen and oxygen analogues. J Biol Chem 21, 10906-10912.
Burger RM, Peisach J, Horwitz SB. 1981 Activated bleomycin. A transient complex of drug, iron, and oxygen that degrades DNA. J Biol Chem 25, 11636-11644.
Burger RM, Horwitz SB, Peisach J. 1985 Stimulation of iron (II) bleomycin activity by phosphate-containing compounds. Biochemistry 24, 3623-3629.
Caspary WJ, Niziak C, Lanzo DA, Friedman R, Bachur NR. 1979 Bleomycin A2: A ferrous oxidase. Mol Pharmacol 16, 256-260.
Holmberg CG, Laurell C-B. 1951 Investigations in serum copper III. Coeruloplasmin as an enzyme. Acta Chem Scand 5, 476-480.
Lown JW, Joshua AV, Chen H-H. 1982 Reactive oxygen species leading to lipid peroxidation and DNA lesions implicated in the cytotoxic action of certain antitumour antibiotics. In: McBrien DCH, Slater TF, eds. Free Radicals, Lipid Peroxidation and Cancer. London: Academic Press; 305-320.
Peisach J, Levine WG. 1963 On the mechanism of ceruloplasmin-catalyzed oxidations. Biochim Biophys Acta 77, 615-628.
Rice EW. 1962 Standardization of ceruloplasmin activity in terms of international enzyme units. Anal Biochem 3, 452-456.
Sausville EA, Peisach J, Horwitz SB 1976. A role for ferrous ion and oxygen in the degradation of DNA by bleomycin. Biochem Biophys Res Commun 73, 814-822.
Sausville EA, Stein RW, Peisach J, Horwitz SB 1978. Effect of chelating agents and metal ions on the degradation of DNA by bleomycin and Fe(II). Biochemistry 17, 2746-2754.
Shields H, McGlumphy C. 1984 The orientation of the ligands in Fe(III)-bleomycin intercalated with DNA. Biochim Biophys Acta 800, 277-281.
Suzuki H, Nagai K, Yamaki H, Tanaka N, Umezawa H. 1969. On the mechanism of action of bleomycin: Scission of DNA strands in vitro and in vivo. J Antibiot 22, 446-448.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Løvstad, R.A. Interaction of p-phenylenediamine with the iron-bleomycin complex. Biometals 12, 233–236 (1999). https://doi.org/10.1023/A:1009298914400
Issue Date:
DOI: https://doi.org/10.1023/A:1009298914400