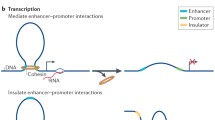
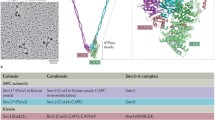
Abstract
Structural maintenance of chromosomes (SMC) family proteins play critical roles in chromosome structural changes. SMC proteins are known to be involved in two major chromosome structural organization events required for mitotic segregation of chromosomes: mitotic chromosome condensation and sister chromatid cohesion. In eukaryotes, two separate sets of SMC heterodimers form the cores of two distinct multiprotein complexes termed ‘condensin’ and ‘cohesin’, each specialized for condensation or cohesion, respectively. It is clear that both condensin and cohesin are conserved in mammals, including humans. The mammalian complexes demonstrate dynamic changes in intracellular distribution in a cell cycle-dependent manner. At any point in the cell cycle, the intracellular localization of the majority of mammalian cohesin and condensin appears to be complementary. Cohesin is associated with chromatin in interphase, while condensin is largely cytoplasmic. Similarly, in mitosis, cohesin is mostly excluded from chromosomes while condensin is distinctly bound to them. Cell cycle-dependent targeting of the two complexes appears to play a major role in regulating their cell cycle-specific activities, and how this redistribution is controlled is an area of active research. Finally, there is evidence that SMC proteins may be involved in DNA recombination and repair. This review focuses on what we have learned about SMC family proteins in humans and other mammalian species in comparison to those in lower eukaryotes. The authors present their own views with regard to some of the major outstanding questions surrounding the nature and functions of the SMC family of proteins.
Similar content being viewed by others
References
Akhmedov AT, Frei C, Tsai-P£ugfelder M, Kemper B, Gasser SM, Jessberger R (1998) Structural maintenance of chromosomes protein C-terminal domains bind preferentially to DNA with secondary structure. J Biol Chem 273: 24088–24094.
Bhat MA, Philp AV, Glover DM, Bellen HJ (1996) Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 87: 1103–1114.
Birkenbihl RP, Subramani S (1992) Cloning and chara-cterization of rad21 an essential gene of Schizosac-charomyces pombe involved in DNA double-strand-break repair. Nucl Acids Res 25: 6605–6611.
Blat Y, Kleckner N (1999) Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259.
Chuang, PT, Albertson DG, Meyer BJ (1994) DPY-27: a chromosome condensation protein homolog that regulates C. elegans dosage compensation through association with X chromosome. Cell 79: 459–474.
Chuang PT, Lieb JD, Meyer BJ (1996) Sex-speci¢c assembly of a dosage compensation copex on the nematode X chromosome. Science 274: 1736–1739.
Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M (1998) An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93: 1067–1076.
Cobbe N, Heck MM (2000) Review: SMCs in the world of chromosome biology from prokaryotes to higher eukaryotes. J Struct Biol 129: 123–143.
Cohen-Fix O, Peters J.-M, Kirschner MW, Koshland D (1996) Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev 10: 3081–3093.
Collas P, Le Guellec K, Taske¨ n K (1999) The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis. J Cell Biol 147 1167–1179.
Cubizolles F, Legagneux V, Le Guellec R et al. (1998) pEg7, a new Xenopus protein required for mitotic chromosome condensation in egg extracts. J Cell Biol 143 1437–1446.
Darwiche N, Freeman LA, Strunnikov A (1999) Characterization of the components of the putative mammalian sister chromatid cohesion complex. Gene 233: 39–47.
de la Barre A-E, Gerson V, Gout S, Creaven M, Allis CD, Dimitrov S (2000) Core histone N-termini play an essential role in mitotic chromosome condensation. EMBO J 19: 379–391.
Eide T, Coghlan S, Orstavik C et al. (1998) Molecular cloning, chromosomal localization, and cell cycle-dependent subcellular distribution of the A-kinase anchoring protein, AKA95. Exp Cell Res 238: 305–316.
Eijpe M, Heyting C, Gross B, Jessberger R (2000) Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 113: 673–682.
Fousteri MI, Lehmann AR (2000) A novel SMC protein complex in Schizosaccharomyces pombe contains the Rad18 DNA repair protein. EMBO J 19: 1691–1702.
Freeman L, Aragon-Alcaide L, Strunnikov A (2000) The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J Cell Biol 149: 811–824.
Guacci V, Koshland D, Strunnikov A (1997) A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91: 47–57.
Hendzel MJ, Wei Y, Mancini MA et al. (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106: 348–360.
Hirano T (1999) SMC-mediated chromosome mechanics: a conserved scheme from bacteria to vertebrates? Genes Dev 13: 11–19.
Hirano T, Mitchison TJ (1994) A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79: 449–458.
Hirano T, Kobayashi R, Hirano M (1997) Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E, and a Xenopus homolog of the Drosophila Barren protein. Cell 89: 511–521.
Hopfner K-P, Karcher A, Shin DS et al. (2000) Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101: 789–800.
Jessberger R, Riwar B, Baechtold H, Akhmedov AT (1996) SMC proteins constitute two subunits of the mammalian recombination complex RC-1. EMBO J 15: 4061–4068.
Kimura K, Hirano T (1997) ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell 90: 625–634.
Kimura K, Hirano T (2000) Dual roles of the 11S regulatory subcomplex in condensin functions. Proc Natl Acad Sci USA 97: 11972–11977.
Kimura K, Hirano M, Kobayashi R, Hirano T (1998) Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 282: 487–490.
Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR (1999) 13S condensin actively reconfigures DNA by introducing global positive writhe: implication for chromosome condensation. Cell 98: 239–248.
Klein F, Mahr P, Galova M et al. (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98: 91–103.
Koshland D, Strunnikov A (1996) Mitotic chromosome condensation. Annu Rev Cell Dev Biol 12: 305–333.
Lieb JD, Albrecht MR, Chuang P-T, Meyer BJ (1998) MIX-1: an essential component of the C. elegans mitotic machinery executes X chromosome dosage compensation. Cell 92: 265–277.
Losada A, Hirano M, Hirano T (1998) Identi¢cation of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12: 1986–1997.
Losada A, Yokochi T, Kobayashi R, Hirano T (2000) Identification and characterization of SA/Scc3p subunits in the Xenopus and human cohesin complexes. J Cell Biol 150: 405–416.
Megee PC, Koshland D (1999) A functional assay for centromere-associated sister chromatid cohesion. Science 285: 254–257.
Megee PC, Mistrot C, Guacci V, Koshland D (1999) The centromeric sister chromatid cohesion site directs Mcd1p binding to adjacent sequences. Mol Cell 4: 445–450.
Melby TE, Ciampaglio CN, Briscoe G, Erickson HP (1998) The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: Long, antiparallel coiled coils, folded at a flexible hinge. J Cell Biol 142: 1595–1604.
Meyer BJ, Casson LP (1986) Caenorhabditis elegans com-pensates for the difference in X chromosome dosage between the sexes by regulating transcript levels. Cell 91: 35–46.
Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: Chromosomal proteins that prevent premature separation of sister chromatids. Cell 91: 35–45.
Nasmyth K, Peters J-M, Uhlmann F (2000) Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288: 1379–1384.
Parisi S, McKay MJ, Molnar M et al. (1999) Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol 19: 3515–3528.
Pezzi N, Prieto I, Kremer L et al. (2000) STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3–related genes flanking the Williams-Beuren syndrome deletion. FASEB J 14: 581–592.
Rocques PJ, Clark J, Ball S, Crew J et al. (1995) The human SB1.8 gene (DXS423E) encodes a putative chromosome segregation protein conserved in lower eukaryotes and prokaryotes. Hum Mol Genet 4: 243–249.
Saitoh N, Goldberg IG, Wood ER, Earnshaw WC (1994) ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J Cell Biol 127: 303–318.
Saka Y, Sutani T, Yamashita Y et al. (1994) Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for cromosome condensation and segregation in mitosis. EMBO J 13: 4938–4952.
Schmiesing JA, Ball AR, Gregson HC, Alderton J, Zhou S, Yokomori K (1998) Identification of two distinct human SMC protein complexes involved in mitotic chromosome dynamics. Proc Natl Acad Sci USA 95: 12906–12911.
Schmiesing JA, Gregson HC, Zhou S, Yokomori K (2000) A human condensin complex containing hCAP-C/hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol Cell Biol 20: 6996–7006.
Shimizu K, Shirataki H, Honda T, Minami S, Takai Y (1998) Complex formation of SMAP/KAP3, a KIF3A/B ATPase motor-associated protein, with a human chromosome-associated polypeptide. J Biol Chem 273: 6591–6594.
Steen RL, Cubizolles F, Le Guellec K, Collas P (2000) A kinase-anchoring protein (AKAP)95 recruits human chromosome-associated protein (hCAP)-D2/Eg7 for chromosome condensation in mitotic extract. J Cell Biol 149: 531–536.
Strick R, Laemmli UK (1995) SARs are cis DNA elements of chromosome dynamics: synthesis of a SAR repressor protein. Cell 83: 1137–1148.
Strunnikov AV, Jessberger R (1999) Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur J Biochem 263: 6–13.
Strunnikov AV, Larionov VL, Koshland D (1993) SMC1: an essential yeast gene encoding a putative head-rod-tail protein is required for nuclear division and defines a new ubiquitous protein family. J Cell Biol 123: 1635–1648.
Strunnikov AV, Hogan E, Koshland D (1995) SMC2, a Sac-charomyces cerevisiae gene essential for chromosome and condensation, defines a subgroup within the SMC family. Genes Dev 9: 587–599.
Stursberg S, Riwar B, Jessberger R (1999) Cloning and characterization of mammalian SMC1 and SMC3 genes and proteins, components of the DNA recombination complexes RC-1. Gene 228: 1–12.
Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters J-M (2000) Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol 151: 749–761.
Sutani T, Yanagida M(1997) DNA renaturation activity of the SMC complex implicated in chromosome condensation. Nature 388: 798–801.
Sutani T, Yuasa T, Tomonaga T, Dohmae N, Takio K, Yanagida M (1999) Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev 13: 2271–2283.
Tanaka T, Cosma MP, Wirth K, Nasmyth K (1999) Identification of cohesin association sites at centromeres and along chromosome arms. Cell 98: 847–858.
Tomonaga T, Nagano K, Kawasaki Y et al. (2000) Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev 14: 2757–2770.
Toth A, Ciosk R, Uhlmann F, Galova M, Schleiffer A, Nasmyth K (1999) Yeast cohesin complex requires a con-served protein, Eco1p (Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev 13: 320–333.
Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400: 37–42.
Waizenegger IC, Hauf S, Meinke AM, Peters J-M (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410.
Wang Z, Castan¬ o IB, De Las Pen¬ as A, Adams C, Christman MF (2000) Pol k: a DNA polymerase required for sister chromatid cohesion. Science 289: 774–779.
Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD (1999) Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 97: 99–109.
Rights and permissions
About this article
Cite this article
Ball, A.R., Yokomori, K. The structural maintenance of chromosomes (SMC) family of proteins in mammals. Chromosome Res 9, 85–96 (2001). https://doi.org/10.1023/A:1009287518015
Issue Date:
DOI: https://doi.org/10.1023/A:1009287518015