Abstract
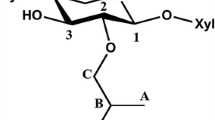
Polymeric xylan can be reacted with propylene oxide (PO) in aqueous alkali homogeneously. Since xylan is isolated from biomass in aqueous alkaline solution, an ‘in-line’ modification with PO as part of the isolation protocol, is most practical. Hydroxypropyl xylan (HPX) is a low molecular weight, branched, water-soluble polysaccharide with low intrinsic viscosity and thermoplasticity. Following peracetylation of HPX in formamide solution, water-insoluble acetoxypropyl xylan (APX) is formed that is also thermoplastic but no longer water soluble. The glass transition temperature (T g) of APX varies in relation to degree of substitution with hydroxypropyl groups (DSPO), and this is found to decline from 160 to 70°C as DSPO rises from 0.2 to 2.0. At a temperature above the T g of HPX a molecular reorganization is noted, and a faint transition due to melting (T m) is observed at 205°C. HPX thermally degrades with a weight loss maximum at 317°C, or approximately 60°C below that of a corresponding cellulose derivative. HPX forms clear films when solvent cast from aqueous solution. Films are higher in ultimate tensile strength and lower in toughness than corresponding cellulose derivative films. The properties of HPX and APX derivatives qualify this material as a potential biodegradable and thermoplastic additive to melt-processed plastics. Blend characteristics with polystyrene reveal a shear-thinning effect in melt and a plasticization effect in solid state.
Similar content being viewed by others
References
Aspinall, G. O. (1959) Structural chemistry of the hemicelluloses. Advan. Carbohydrate Chem. 14, 429-468.
Carson, J. F. and Maclay, W. D. (1948) Esters of lima bean pod and corn cob hemicelluloses. J. Amer. Chem. Soc. 70, 293-295.
Carson, J. F. and Maclay, W. D. (1946) The acylation of polyuronides with formamide as a dispersing agent. J. Amer. Chem. Soc. 68, 1015-1017.
Chesson, A. (1988) Lignin-polysaccharide complexes of the plant cell wall and their effect on microbial degradation in the rumen. Animal Feed Sci. Technol. 21, 219-228.
Croon, I. and Donetzhuber, A. (1963) Xanthation and dexanthation of some hemicellulose fractions. Tappi 46, 648-651.
Eriksson, O. and Lindgren, B. O. (1977) About the linkage between lignin and hemicelluloses in wood. Svensk Papperstidn. 80, 59-63.
Glasser, W. G., Ravindran, G., Jain, R. K., Samaranayake, G. and Todd, J. (1995) Comparative enzyme biodegradability of xylan, cellulose, and starch derivatives. Biotechnol. Progr. 11, 552-557.
Glasser, W. G., Kaar, W. E., Jain, R. K. and Sealey, J. E. (2000) Isolation options for noncellulosic heteropolysaccharides. Cellulose 7(3), 299-317.
Glaudemans, C. P. J. and Timell, T. E. (1958) The polysaccharides of white birch (Betula papyrifera). Svensk Papperstidn. 61, 1-9.
Goring, D. A. I. (1968) Thermal softening of lignin, hemicellulose, and cellulose. Pulp Paper Mag. Can. 64(12), T517-T527.
Hirst, E. L. (1962) The chemical structure of the hemicelluloses. Pure Appl. Chem. 5, 53-66.
Horio, M. and Imamura, R. (1964) Crystallographic study of xylan from Wood. J. Polym. Sci. A-2, 627-644.
Irvine, G. M. (1984) The glass transition of lignin and hemicellulose and their measurement by differential thermal analysis. Tappi 67(5), 118-121.
Jeffries, T. W. (1990) Biodegradation of lignin-carbohydrate complexes. Biodegradation 1, 163-176.
Kim, H. R. and Eliasson, A.-C. (1993) The influence of molar substitution on the thermal transition properties of hydroxypropyl potato starches. Carbohydrate Polym. 22, 31-35.
Koshijima, T., Timell, T. E. and Zinbo, M. (1965) The number-average molecular weight of native hardwood xylans. J. Polym. Sci. C-11, 265-279.
Lindberg, B. (1962) Recent advances in methods of isolating and purifying hemicelluloses. Pure Appl. Chem. 5, 67-75.
Majewicz, T. G. and Podlas, T. J. (1993) Cellulose ethers, in Encycl. Chemical Technology. M. Howe-Grant, (ed.). 4th edn, vol. 5, Wiley, NY, pp. 541-563.
Marchessault, R. H. and Settineri, W. J. (1964) Some comments on the crystallography of xylan hydrate. J. Polym. Sci. Polym. Letter 2, 1047-1051.
Marchessault, R. H. and Liang, C. Y. (1962) The infrared spectra of crystalline polysaccharides. VIII. xylans. J. Polym. Sci. 59, 357.
O'Malley, J. J. and Marchessault, R. H. (1966) Characterization of graft copolymers of methylated xylan and polystyrene. J. Phys. Chem. 70, 3235-3240.
Ramiah, M. V. and Goring, D. A. I. (1965) The thermal expansion of cellulose, hemicellulose, and lignin. J. Polym. Sci. C-11, 27-48.
Rexen, F. (1979) Low-quality forages improve with alkali treatment. Feedstuffs 51(42), 33-34.
Rials, T. G. and Glasser, W. G. (1988) Thermal and dynamic mechanical properties of hydroxypropyl cellulose films. J. Appl. Polym. Sci. 36, 749-758.
Russo, J. R. (1976) Xylitol: anti-carie sweetener? Food Engng 48(4), 77-79.
Salmen, L. and Olsson, A.-M. (1998) Interaction between hemicelluloses, lignin and cellulose: structure-property relationships. J. Pulp Paper Sci. 24(3), 99-103.
Schmorak, J. and Adams, G. A. (1957) The preparation and properties of carboxymethylxylan. Tappi 40, 378-383.
Schweiger, R. G. (1976) Nitrite esters of polyhydroxy polymers. J. Org. Chem. 41, 90-93.
Timell, T. E. (1964) Wood hemicellulose: Part I. Advan. Carbohydrate Chem. 19, 247-302.
Timell, T. E., Glaudemans, C. P. J. and Gillham, J. K. (1959) Recent studies on the polysaccharides of white birch and other hardwoods. Tappi 42, 623-634.
Vincendon, M. (1998) Xylan derivatives: Aromatic carbamates. Makromol. Chem. 194, 321-328; Xylan derivatives: Benzyl ethers, synthesis, and characterization. J. Appl. Polym. Sci. 67, 455-460.
Whistler, R. L. and Daniel, J. R. (1983) Starch, In Encycl. Chemical Technology. H. F. Mark, D. F. Othmer, C. G. Overberger and G. T. Seaborg, (eds). 3rd edn, vol. 21, Wiley, NY., pp. 492-507.
Whistler, R. L. (1950) Xylan. Advan. Carbohydrate Chem. 5, 269-290.
Wilkie, K. C. B. (1979) The hemicelluloses of grasses and cereals. Advan. Carbohydrate Chem. Biochem. 36, 215-264.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jain, R.K., Sjöstedt, M. & Glasser, W.G. Thermoplastic Xylan Derivatives with Propylene Oxide. Cellulose 7, 319–336 (2000). https://doi.org/10.1023/A:1009260415771
Issue Date:
DOI: https://doi.org/10.1023/A:1009260415771