Abstract
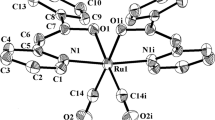
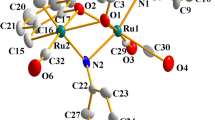
The syntheses of Ru3(CO)9(PTA)3 and Ir4(CO)7(PTA)5 were accomplished through the thermal reactions of Ru3(CO)12 or Ir4(CO)12 with the water-soluble phosphine, PTA(1,3,5-triaza-7-phosphaadamantane). The ruthenium derivative was shown by X-ray crystallography to consist of a triangular Ru3 core with three nearly equal Ru–Ru bonds, with each ruthenium atom bearing an equatorially positioned PTA ligand. In Ir4(CO)7(PTA)5 the iridium atoms define a tetrahedron which is bridged on three edges by CO ligands. One basal iridium atom contains two PTA ligands, while the other two basal and the apical iridium atoms each possess one PTA ligand in their coordination spheres. Although, Ru3(CO)9(PTA)3 is only sparingly soluble in pure water, it is very soluble in aqueous solution of pH<4. Indeed the triruthenium cluster can be extracted reversibly between an aqueous and an organic phase (e.g., CH2Cl2) by changing the pH of the aqueous phase. On the other hand the more highly PTA substituted cluster, Ir4(CO)7(PTA)5, exhibits good solubility in aqueous solution (pH 7 and below) and a variety of organic solvents. Both cluster derivatives are stable in deoxygenated, aqueous solutions for extended period of time (>24 h).
Similar content being viewed by others
REFERENCES
D. J. Darensbourg, J. B. Robertson, D. L. Larkins, and J. H. Reibenspies (1999). Inorg. Chem. 38, 2473.
W. Keim, in A. Mortreux, F. Petir, and D. Reidel (eds.), Industrial Applications of Homogeneous Catalysis (Dordrecht, 1988), p. 338.
B. Cornils and W. A. Herrmann, in B. Cornils and W. A. Herrmann (eds.), Aqueous-Phase Organometallic Catalysis (Wiley-VCH, Weinheim, 1998), p. 583.
(a) F. Joó and A. Bényei (1989). J. Organomet. Chem. 363, C19; (b) A. Bényei and F. Joó (1990). J. Mol. Catal. 58, 151.
J. M. Grosselin, C. Mercier, G. Allmang, and F. Grass (1991). Organometallics 10, 2126.
M. Y. Darensbourg and D. Daigle (1975). Inorg. Chem. 14, 1217.
J. R. DeLerno, L. M. Trefonas, M. Y. Darensbourg, and R. J. Majeste (1976). Inorg. Chem. 15, 816.
E. Fluck, J. E. Förster, J. Weidlein, and E. Hädicke (1977). Z. Naturforsch. Teil B 32B, 499.
K. J. Fisher, E. C. Alyea, and N. Shahnazarian (1990). Phosphorus, Sulfur, Silicon Relat. Elem. 48, 37.
(a) D. J. Darensbourg, F. Joó, M. Kannisto, A. Kathó, and J. H. Reibenspies (1992). Organometallics 11, 1990; (b) D. J. Darensbourg, F. Joó, M. Kannisto, A. Kathó, J. H. Reibenspies, and D. J. Daigle (1994). Inorg. Chem. 33, 200.
D. J. Daigle (1998). Inorg. Synth. 32, 40.
D. J. Darensbourg, T. J. Decuir, and J. H. Reibenspies, in I. T. Horváth and F. Joó (eds.), Aqueous Organometallics Chemistry and Catalysis, NATO ASI Series 3, High Technology (Kluwer, Dordrecht, The Netherlands, 1995), pp. 61-77.
M. R. Churchill, F. J. Hollander, and J. P. Hutchinson (1977). Inorg. Chem. 16, 2655.
G. Lavigne and H. D. Kaesz (1984). J. Am. Chem. Soc. 106, 4647.
B. Fontal, J. Orlewski, C. C. Santini, and J. M. Basset (1986). Inorg. Chem. 25, 4322.
I. S. Butler, Zhen H. Xu, D. J. Darensbourg, and M. Pala (1987). J. Raman Spectr. 18, 357.
D. R. Tyler, R. A. Levenson, and H. B. Gray (1978). J. Am. Chem. Soc. 100, 7888.
D. J. Darensbourg and B. J. Baldwin-Zuschke (1981). Inorg. Chem. 20, 3846.
M. R. Churchill and J. P. Hutchinson (1978). Inorg. Chem. 17, 3528.
M. R. Churchill and J. P. Hutchinson (1980). Inorg. Chem. 19, 2765.
V. Albano, P. Bellon, and V. Scatturin (1967). Chem. Commun. 730.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Darensbourg, D.J., Beckford, F.A. & Reibenspies, J.H. Water-Soluble Organometallic Compounds. 8[1]. Synthesis, Spectral Properties, and Crystal Structures of 1,3,5-Triaza-7-phosphaadamantane (PTA) Derivatives of Metal Carbonyl Clusters: Ru3(CO)9(PTA)3 and Ir4(CO)7(PTA)5. Journal of Cluster Science 11, 95–107 (2000). https://doi.org/10.1023/A:1009060614412
Issue Date:
DOI: https://doi.org/10.1023/A:1009060614412