Abstract
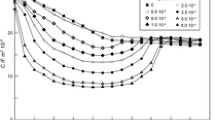
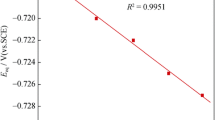
Effects of concentrations of ammonia (0.3–5.8 M) and supporting electrolytes (NaF, NaClO4; 0.1–0.5 M) on the kinetics of electroreduction of ammonia complexes of cobalt(II) at a dropping mercury electrode are studied. Most experiments are performed with low concentrations of cobalt(II) complexes (1 × 10–5 to 2 × 10–5 M) in the absence of a polarographic maximum. The dependence of the half-wave potential of the reversible cathodic wave pertaining to the reduction of ammonia complexes of cobalt(II) on the concentration of ammonia molecules is obtained. It is found from the dependence that, at ammonia concentrations of 0.5–2.6 M, the slow electrochemical stage involves predominantly complexes Co(NH3)2 2+. At higher ammonia concentrations, the stage involves complexes Co(NH3) k 2+ (k > 2), which form in preceding chemical stages from complexes Co(NH3) i 2+ (i = 3–6) that are predominant in solution. Values of the diffusion coefficients for complexes Co(NH3) i 2+, apparent transfer coefficients, and rate constant of the process of electroreduction of ammonia complexes of cobalt(II) are determined. The reasons for the complicating effect the insoluble products of reduction of cobalt(II) complexes have on the shape of polarographic waves are discussed.
Similar content being viewed by others
REFERENCES
Kovalenko, P.N. and Nadezhina, L.S., Zh. Org. Khim., 1952, vol. 22, p. 740.
Eriksrud, E., J. Electroanal. Chem., 1975, vol. 60, p. 53.
Astakhova, R.K., Balushkina, S.R., Kravtsov, V.I., and Peganova, N.V., Elektrokhimiya, 1999, vol. 35, p. 1395.
Heyrovsk, J. and Kůta, J., Základy Polarografie (Fundamentals of Polarography), Prague: Nakladatelstvi Czeskoslovenske Akademie Ved., 1962.
Bjerrum, J., Metal Ammine Formation in Aqueous Solution, København: I Kommission hos E. Munksgaard, 1957.
Paoletti, P., Stern, J.H., and Vacca, A., J. Phys. Chem., 1965, vol. 69, p. 3759.
Weaver, M.J. and Satterberg, T.L., J. Phys. Chem., 1977, vol. 81, p. 1772.
Gubbeli, A.O., Hébert, J., Taillon, R., and Cótc, P.A., Helv. Chim. Acta, 1970, vol. 53, p. 1229.
Tur'yan, Ya.I., Dokl. Akad. Nauk SSSR, 1957, vol. 113, p. 169.
Mairanovskii, S.G., Elektrokhimiya, 1967, vol. 3, p. 1434.
Fleischamn, M., Harrison, J.A., and Thirsk, H.R., Trans. Faraday Soc., 1965, vol. 61, p. 2742.
Ivanov, V.F. and Iofa, Z.A., Dokl. Akad. Nauk SSSR, 1961, vol. 140, p. 1368.
Ivanov, V.F. and Iofa, Z.A., Zh. Fiz. Khim., 1962, vol. 36, p. 1080.
Basolo, F. and Pearson, R., Mechanisms of Inorganic Reactions: A Study of Metal Complexes in Solution, New York: Wiley, 1967.
Kravtsov, V.I., Ravnovesie i kinetika elektrodnykh reaktsii kompleksov metallov (The Equilibrium and Kinetics of Electrode Reactions Involving Metal Complexes), Leningrad: Khimiya, 1985.
Meites, L. and Israel, Y., J. Am. Chem. Soc., 1961, vol. 83, p. 4903.
Russell, C.D., J. Electroanal. Chem., 1963, vol. 6, p. 490.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Astakhova, R.K., Kravtsov, V.I. & Peganova, N.V. Kinetics and Mechanism of Electroreduction of Ammonia Complexes of Cobalt(II) on a Dropping Mercury Electrode. Russian Journal of Electrochemistry 37, 161–169 (2001). https://doi.org/10.1023/A:1009031908614
Issue Date:
DOI: https://doi.org/10.1023/A:1009031908614