Abstract
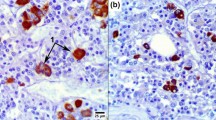
PEPCK/bGH transgenic mice have very high blood levels of foreign GH, and prominent reproductive disturbances, especially in females. To obtain a deeper insight into the causes of these abnormalities, pituitaries of PEPCK/bGH transgenics were studied by immunocytochemistry, electron microscopy and in situ hybridization (ISH) techniques. Pituitary weights were significantly reduced (P < 0.05) in transgenic males, while in transgenic females they were increased without reaching significance compared to nontransgenic controls. In both sexes, GH cells were inhibited, as previously described in other lines of GH transgenic mice. In females, PRL cells were increased by 37% compared to controls. Ultrastructurally, the lactotrophs had characteristics of stimulation and PRL mRNA was increased by 35%. In males the increase in the number of PRL immunoreactive cells was not significant, the PRL mRNA signal did not differ from controls, and there were no changes in their ultrastructure. Only in females ACTH cells were significantly reduced (P < 0.05) in number and unchanged in males; however, POMC mRNA signal was increased in both genders and reached significance (P < 0.05) in males. In females, but not in males, the percentage of LH cells was lower than in control mice. In conclusion, the high blood bGH levels induced sex related changes in transgenic mice from the present line. The infertility of PEPCK/bGH transgenic females may be attributed to lactotroph hyperplasia and marked reduction in number of gonadotrophs.
Similar content being viewed by others
References
Aguilar RC, Fernandez HN, Dellacha JM, Calandra RS, Bartke A and Turyn D (1992) Identification of somatogenic binding sites in liver microsomes from normal mice and transgenic mice expressing human growth hormone gene. Life Sci 50:615–620.
Atkin SL, Landolt AM, Foy P, Jeffreys RV, Hipkin L and White MC (1994) Effects of insulin-like growth factor-I on growth hormone and prolactin secretion and cell proliferation of human somatotrophinomas and prolactinomas in vitro. Clin Endocrinol 41: 503–509.
Bartke A, Cecim M, Tang K, Steger RW, Chandrashekar V and Turyn D (1994) Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc Soc Exp Biol Med 206:345–359.
Bartke A, Steger RW, Hodges SL, Parkening TA, Collins TJ, Yun JS and Wagner TE (1988) Infertility in transgenic female mice with human growth hormone expression: evidence for luteal failure. J Exp Zool 248:121–124.
Bartke A, Naar EM, Johnson L, May MR, Cecim M, Yun JS and Wagner TE (1992) Effects of expression of human or bovine growth hormone genes on sperm production and male reproductive performance in four lines of transgenic mice. J Reprod Fertil 95:109–118.
Brolin SE and Theander G (1945) Quantitative investigations on the anterior pituitary body of castrate rats with reference to the absolute cell conditions and their significance. Acta Anat 1: 72–94.
Cecim M, Alvarez-Sanz M, van de Kar L, Milton S and Bartke A (1996) Increased plasma corticosterone levels in bovine growth hormone (bGH) transgenic mice: effects of ACTH, GH and IGFI on in vitroadrenal corticosterone production. Transgenic Res 5: 187–192.
Cecim M, Ghosh PK, E squifino AI, Began T, Wagner TE, Yun JS and Bartke A (1991) Elevated corticosterone levels in transgenic mice expressing human or bovine growth hormone genes. Neuroendocrinology 53:313–316.
Cecim M, Kerr J and Bartke A (1995) Infertility in transgenic mice overexpressing the bovine growth hormone gene: Luteal failure secondary to prolactin deficiency. Biol Reprod 52: 1162–1166.
Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C and Bloch BA (1988)Growth hormone-deficient dwarfism in the rat: A new mutation. J Endocrinol 119:51–58.
Denef C and Andries M (1983) Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology 112:813–822.
Felix IA, Horvath E, Kovacs K, Smyth HS, Killinger DW and Vale J (1986) Mammosomatotroph adenoma of the pituitary associated with gigantism and hyperprolactinemia. A morphological study including immunoelectron microscopy. Acta Neuropathol 71:76–82.
Floderus S (1944) Untersuchugen uber den Bau der menschlichen Hypophyse mit besonderer Berucksichtigung der quantitaven mikromorphologischen Verhaltnisse. Acta Pathol Microbiol Scand(Suppl) 53: 1–276.
Goodyer CG, DeStephano L, Guyda HJ, and Posner BI (1984) Effects of insulin-like growth factors on adult male rat pituitary function in tissue culture. Endocrinology 115:1568–1576.
Goodyer CG, Marcovitz S, Hardy J, Lefebvre Y, Guyda HJ and Posner BI (1986) Effect of insulin-like growth factors on human foetal, adult normal and tumour pituitary function in tissue culture. Acta Endocrinol 112:49–57.
Hartree AS, Kovacic N and Thomas M (1965) Growth-promoting and luteotrophic activities of human growth hormone. J Endocrinol 33:249–258.
Hsu SM, Raine L and Fanger H (1981) Use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580.
Hurley DL, Bartke A, Wagner TE, Wee BE and Phelps CJ (1994) Increased hypothalamic somatostatin expression inmice transgenic for bovine or human GH. J Neuroendocrinol 6:539–548.
Lamberts SW, den Holder F and Hofland LJ (1989) The interreationship between the effects of insulin-like growth factor I and somatostatin on growth hormone secretion by normal rat pituitary cells: The role of glucocorticoids. Endocrinology 124: 905–911.
Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL and Palmiter RD (1988) Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123:2827–2833.
Mathiasen JR, Tomogane H and Voogt JL (1992) Serotonininduced decrease in hypothalamic tyrosine hydroxylase activity and corresponding increase in prolactin release are abolished at midpregnancy and by transplants of rat choriocarcinoma cells. Endocrinology 131:2527–2532.
McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM and Hanson RW (1988) Tissuespecific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem 263: 11443–11451.
Naar EM, Bartke A, Majumdar SS, Buonomo FC, Yun JS and Wagner TE (1991) Fertility of transgenic female mice express202 ing bovine growth hormone or human growth hormone variant genes. Biol Reprod 45:178–187.
Nogami H and Takeuchi T (1993) Increased population of nonhormone-producing cells suggests the presence of dysfunctional growth hormone cells in the anterior pituitary gland of the spontaneous dwarf rat. Neuroendocrinology 57:374–380.
Orian JM, Lee CS, Weiss LM and Brandon MR (1989) The expression of a metallothionein-ovine growth hormone fusion gene in transgenic mice does not impair fertility but results in pathological lesions in the liver. Endocrinology 124:455–463.
Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC and Evans RM (1982) Dramatic growth of mice that develop from eggs microinjected with metallothioneingrowth hormone fusion genes. Nature 300:611–615.
Palmiter RD, Norstedt G, Gelinas RE, Hammer RE and Brinster RL (1983) Metallothionein-human GH fusion genes stimulate growth of mice. Science 222:809–814.
Sagrillo CA and Voogt JL (1992) Mechanisms for the stimulatory effects of opioidergic and serotonergic input signals on prolactin in pregnant rats. Life Sci 50:1479–1489.
Shea BT, Hammer RE and Brinster RL (1987) Growth allometry of the organs in giant transgenic mice. Endocrinology 121: 1924–1930.
Stefaneanu L, Kovacs K and Bartke A (1993a) In situhybridization study of growth hormone, prolactin, and proopiomelanocortin mRNAs in adenohypophyses of mice transgenic for human growth hormone. Endocr Pathol 4:73–78.
Stefaneanu L, Kovacs K, Bartke A, Mayerhofer A and Wagner TE (1993b) Pituitary morphology of transgenic mice expressing bovine growth hormone. Lab Invest 68:584–591.
Stefaneanu L, Kovacs K, Horvath E, Losinski NE, Mayerhofer A, Wagner TE and Bartke A (1990) An immunocytochemical and ultrastructural study of adenohypophyses of mice transgenic for human growth hormone. Endocrinology 126:608–615.
Stefaneanu L, Powell-Braxton L, Won W, Chandrashekar V, Bartke A (1999) Somatotroph and lactotroph changes in the adenohyprophyses of mice with disrupted insulin-like growth factor I gene. Endocrinology(in press).
Stefaneanu L, Rindi G, Horvath E, Murphy D, Polak JM and Kovacs K (1992) Morphology of adenohypophysial tumors in mice transgenic for vasopressin-SV40 hybrid oncogene. Endocrinology 130:1789–1795.
Steger RW, Bartke A and Cecim M (1993) Premature aging in transgenic mice expressing different growth hormone genes. J Reprod Fertil(Suppl) 46:61–75.
Steger RW, Bartke A, Parkening TA, Collins T, Cerven R, Yun JS and Wagner T (1994) Effects of chronic exposure to bovine growth hormone (bGH) on the hypothalamic-pituitary axis in transgenic mice: Relationship to the degree of expression of the PEPCK/bGH hybrid gene. Transgenics 1:245–253.
Tang K, Bartke A, Gardiner CS, Wagner TE and Yun JS (1993) Gonadotropin secretion, synthesis, and gene expression in human growth hormone transgenic mice and in Ames dwarf mice. Endocrinology 132:2518–2524.
Terada T, Stefaneanu L, Kovacs K and Bartke A (1994) Pituitary morphology in mice transgenic for phosphoenolpyruvate carboxykinase promoter region-human growth hormone fusion gene. Endocrine 2:151–155.
Yamashita S and Melmed S (1986) Insulin-like growth factor I action on rat anterior pituitary cells: Suppression of growth hormone secretion and messenger ribonucleic acid levels. Endocrinology 118:176–182.
Yamashita S and Melmed S (1987) Insulin-like growth factor I regulation of growth hormone gene transcription in primary rat pituitary cells. J Clin Invest 79:449–452.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vidal, S., Stefaneanu, L., Thapar, K. et al. Lactotroph hyperplasia in the pituitaries of female mice expressing high levels of bovine growth hormone. Transgenic Res 8, 191–202 (1999). https://doi.org/10.1023/A:1008958807096
Issue Date:
DOI: https://doi.org/10.1023/A:1008958807096