Abstract
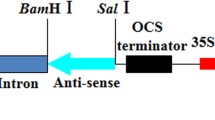
Two modified plum pox virus (PPV) coat protein (CP) gene constructs, designed to reduce putative biological risks associated with heteroen capsidation, were integrated into Nicotiana benthamiana plants. The first one contained a deletion of the nucleotides encoding for the DAG amino acid triplet involved in virus aphid-transmission. In the second one, the first 420 nucleotides of the PPV CP gene were removed. We present here the analysis and the selection throughout the generations of PPV-resistant transgenic lines containing these constructs. In most of the lines, a recovery phenotype was observed and was associated with a down-regulation of the transgene products (RNA or protein). We also describe two lines that were highly resistant to PPV. This immunity was correlated with a high number of transgene copies (at least three) and with low or undetectable transgene RNA levels. No heterologous protection was observed against other potyviruses. These characteristics indicate that the described resistance against PPV was RNA-mediated and can be classified as a 'sense suppression' or homology-dependent resistance. Moreover, the production of a highly resistant line containing the PPV CP gene with one third of its 5′ end deleted indicated that this region is not necessary to trigger the plant resistance mechanism(s)
Similar content being viewed by others
References
Atreya, C.D., Raccah, B. and Pirone, T.P. (1990) A point mutation in the coat protein abolishes aphid transmissibity of a potyvirus. Virology 178, 161-5.
Atreya, P.L., Atreya, C.D. and Pirone, T.P. (1991) Amino acid substitution in the coat protein result in loss of insect transmissibility of a plant virus. Proc. Natl Acad. Sci. USA 88, 7887-91.
Atreya, P.L., Lopez-Moya, J.J., Chu, M., Atreya, C.D. and Pirone, T.P. (1995) Mutational analysis of the coat protein N-terminal amino acids involved in potyvirus transmission by aphids. J. Gen. Virol. 76, 265-70.
Baulcombe, D.C. (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8, 1833-44.
Beachy, R.N., Loesh-Fries, S. and Turner, N.E. (1990) Coat protein mediated resistance against virus infection. Annu. Rev. Phytopathol. 28, 451-74.
Berger, P.H., Hunt, A.G., Domier, L.L., Helmann, G.M., Stram, Y., Thornbury, D.W. and Pirone, T.P. (1989) Expression in transgenic plants of a viral gene product that mediates insect transmission of potyvirus. Proc. Natl Acad. Sci. USA 86, 8402-6.
Cassidy, B.G. and Nelson, R.S. (1995) Differences in protection phenotypes in tobacco plants expressing coat protein genes from peanut stripe potyvirus with or without an engineered ATG. Mol. Plant-Microbe Interact. 8, 357-65.
Clark, G.C., Fitchen, J.H. and Beachy, R.N. (1995) Studies of coat protein-mediated resistance to TMV: The PM2 assembly defective mutant confers resistance to TMV. Virology 208, 485-91.
de Carvalho, F., Gheysen, G., Kushnir, S., Van Montagu, M., Inzé, D. and Castresana, C. (1992) Suppression of the β-1,3-glucanase transgene expression in homozygous plants. EMBO J. 11, 2595-602.
Dougherty, W.G., Lindbo, J.L., Smith, H.A., Parks, T.D., Swaney, S. and Proebsting, W.M. (1994) RNA-mediated virus resistance in transgenic plants: Exploitation of a cellular pathway possibly involved in RNA degradation. Mol. Plant-Microbe Interact. 7, 544-52.
Doyle, J.J. and Doyle, J.L. (1990) Isolation of plant DNA from fresh tissue. Focus 12, 13-5.
English, J.J., Mueller, E. and Baucombe, D.C. (1996) Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes. Plant Cell 8, 179-88.
Farell, L.B. and Beachy, R.N. (1992) Review of the use of the GUS gene for analysis of secretory systems. In Gallagher, S.R. ed. GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression, Academic Press, Inc. pp. 127-134.
Farinelli, L. and Malnoe, P. (1993) Coat protein gene-mediated resistance to potato virus Y in tobacco: examination of the resistance mechanisms — Is the transgenic coat protein required for protection? Mol. Plant-Microbe Interact. 6, 284-92.
Flavell, R.B. (1994) Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc. Natl Acad. Sci. USA 91, 3490-6.
Goodwin, J., Chapman, K., Swaney, S., Parks, T.D., Wernsman, E.A. and Dougherty, W.G. (1996) Genetic and biochemical dissection of transgenic RNA-mediated virus resistance. Plant Cell 8, 95-105.
Horsch, R.B., Fry, J.E., Hoffmann, N.L., Eicholtz, D., Rogers, S.G. and Fraley, R.T. (1985) A simple and general method for transferring genes into plants. Science 227, 1229-231.
Kay, R., Chan, A., Daly, M. and McPherson, J. (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236, 1299-302.
Kerlan, C. and Dunez, J. (1979) Différenciation biologique et sérologique de souche de virus de la sharka. Ann. Phytopathologie 11, 241-50.
Laemmli, E.K. (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227, 680-5.
Laimer da Camara Machado, M., da Camara Machado, A. and Hanzer, V. (1992) Regeneration of transgenic plants of Prunus armeniaca containing the coat protein gene of plum pox virus. Plant Cell Reports 11, 25-9.
Lecoq, H., Ravelonandro, M., Wipf-Scheibel, C., Monsion, M., Raccah, B. and Dunez, J. (1993) Aphid transmission of an aphid non-transmissible strain of zucchini yellow mosaic potyvirus from transgenic plants expressing the capsid protein of plum pox potyvirus. Mol. Plant-Microbe Interact. 6, 403-6.
Lindbo, J.A. and Dougherty, W.G. (1992a) Pathogen-derived resistance to a potyvirus: immune and resistant phenotypes in transgenic tobacco expressing altered forms of a potyvirus coat protein nucleotide sequence. Mol. Plant-Microbe Interact. 5, 141-53.
Lindbo, J.A. and Dougherty, W.G (1992b) Untranslatable transcripts of the tobacco etch virus coat protein gene sequence can interfere with tobacco etch replication in transgenic plants and protoplasts. Virology 189, 725-33.
Lindbo, J.A., Silva-Rosales, L., Proebsting, W.M. and Dougherty, W.G. (1993) Inhibition of a highly specific antiviral state in transgenic plants: Implications for regulation of gene expression and virus resistance. Plant Cell 5, 1749-59.
Lomonossoff, G.P. (1993) Pathogen-derived resistance to plant virus. Annu. Rev. Phytopathol. 33, 323-43.
Maiss, E., Timpe, U., Briske, A., Jelkmann, W., Casper, R., Himmler, G., Mattanovich, D. and Katinger, H.W.D. (1989) The complete nucleotide sequence of plum pox virus RNA. J. Gen. Virol. 70, 513-24.
Mante, S., Morgens, P.H., Scorza, R., Cordts, J.M. and Callahan, A.M. (1991) Agrobacterium-mediated transformation of plum (Prunus domestica L.) hypocotyl slices and regeneration of transgenic plants. Bio/Technology 9, 752-8.
Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bio-assays with tobacco tissue. Physiol. Plant 15, 473-97.
Nejidat, A. and Beachy, R.N. (1990) Transgenic tobacco plants expressing a coat protein gene of tobacco mosaic virus are resistant to some other tobamoviruses. Mol. Plant-Microbe Interact. 3, 247-51.
Powell, A.P., Nelson, R.S., De, B., Hoffmann, N., Rogers, S.G., Fraley, R.T. and Beachy, R.N. (1986) Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232, 738-43.
Powell, P.A., Sanders, P.R., Tumer, N., Fraley, R.T. and Beachy, R.N. (1990) Protection against tobacco mosaic virus infection in transgenic plants requires accumulation of coat protein rather than coat protein RNA sequences. Virology 173, 124-30.
Ravelonandro, M., Monsion, M., Teycheney, P.Y., Delbos, R. and Dunez, J. (1992) Construction of a chimeric viral gene expressing plum pox coat protein. Gene 120, 167-73.
Ravelonandro, M., Monsion, M., Delbos, R. and Dunez, J. (1993) Variable resistance to plum pox virus and potato virus Y infection in transgenic Nicotiana plants expressing plum pox virus coat protein. Plant Sci. 91, 157-69.
Register, J.C., Powell, P.A., Nelson, R.S. and Beachy, R.N. (1989) Genetically engineered cross protection against TMV interferes with initial infection and long distance spread of the virus, in Molecular Biology of Plant-Pathogen Interactions. New York, USA. Alan R. Liss, pp. 269-281.
Register, J.C. and Nelson, R.S. (1992) Early events in plant virus infection: relationships with genetically engineered protection and host gene resistance. Sem. Virol. 3, 441-51.
Regner, F., da Machado, A., Laimer da Machado, M., Steinkellner, H., Mattanovitch, D., Hanzer, H., Weiss, H. and Katinger, H. (1992) Coat protein mediated resistance to plum pox virus in Nicotiana clevelandii and N. benthamiana. Plant Cell Rep. 11, 30-3.
Salomon, R. and Bernardi, F. (1995) Inhibition of viral aphid transmission by the N-terminus of the maize dwarf mosaic virus coat protein. Virology 213, 676-9.
Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: a Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press).
Sanford, J.C. and Johnston, S.A. (1985) The concept of parasite-derived resistance-deriving resistance genes from the parasite's own genome. J. Theor. Biol. 113, 395-405.
Scholthof, K.B.G., Scholthof, H.B. and Jackson, A.O. (1993) Control of plant virus diseases by pathogen-derived resistance in transgenic plants. Plant Physiol. 102, 7-12.
Scorza, R., Ravelonandro, M., Callahan, A.M., Cordts, J.M., Fuchs, M. and Dunez, J. (1994) Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep. 14, 18-22.
Shukla, D.D. and Ward, C.W. (1989) Identification and classification of potyviruses on the basis of coat protein sequence data and serology. Arch. Virol. 106, 171-200.
Silva-Rosales, L., Linbo, J.A. and Dougherty, W.G. (1994) Analysis of transgenic tobacco plants expressing a truncated form of a potyvirus coat protein nucleotide sequence. Plant Mol. Biol. 24, 929-39.
Smith, H.A., Swaney, S.L., Parks, T.D., Wernsman, E.A. and Dougherty, W.G. (1994) Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of nonessential RNAs. Plant Cell 6, 1441-53.
Teycheney, P.Y., Tavert, G., Delbos, R.P., Ravelonandro, M. and Dunez, J. (1989) The complete nucleotide sequence of plum pox virus RNA (strain D). Nucl. Acids Res. 17, 10115-6.
van der Vlugt, R.A.A., Ruiter, R.K. and Goldbach, R. (1992) Evidence for sense RNA-mediated protection to PVYN in tobacco plants transformed with the viral coat protein cistron. Plant Mol. Biol. 20, 631-9.
Verwoerd, T.C., Dekker, B.M. and Hoekema, A. (1989) A small scale procedure for the rapid isolation of plant RNAs. Nucl. Acids Res. 17, 2362.
Wilson, T.M.A. (1993) Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc. Natl Acad. Sci. USA 90, 3134-41.
Wisniewski, L.A., Powell, P.A., Nelson, R.S. and Beachy, R.N. (1990) Local and systemic spread of tobacco mosaic virus in transgenic tobacco. Plant Cell 2, 559-67.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jacquet, C., Ravelonandro, M., Bachelier, Jc. et al. High resistance to plum pox virus (PPV) in transgenic plants containing modified and truncated forms of PPV coat protein gene. Transgenic Res 7, 29–39 (1998). https://doi.org/10.1023/A:1008851821374
Issue Date:
DOI: https://doi.org/10.1023/A:1008851821374