Abstract
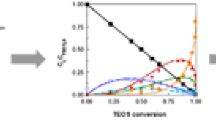
Heat of reaction of the hydrolysis-polymerization process of tetraethyl orthosilicate with water in acidic condition was investigated to clarify the thermodynamic driving force of sol-gel reactions. Heat of reaction was measured using an isoperibol calorimeter by mixing a dilute tetraethyl orthosilicate (TEOS) ethanolic solution with another solution of water, ethanol, and hydrochloric acid. The temperature change of the reaction cell had been measured more than 24 hours after mixing under the quasi-isothermal condition. Large exothermic reaction (12.9 kJ·mol−1 for 1 mole of TEOS) due to the hydrolysis of TEOS was observed. A slow exothermic reaction followed it, and after that, the sol-gel reaction was changed to a small endothermic one.
Similar content being viewed by others
References
D.A. Donatti and D.R. Vollet, J. Non-Cryst. Solids 208, 99 (1996).
D.R. Vollet, D.A. Donatti, and Ibanez Ruiz, J. Sol-Gel Sci. Tech. 15, 5 (1999).
P. Boutron and A. Kaufman, J. Chem. Phys. 68, 5032 (1978).
S. Sjöberg, J. Non-Cryst. Solids 196, 51 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsuoka, J., Numaguchi, M., Yoshida, S. et al. Heat of Reaction of the Hydrolysis-Polymerization Process of Tetraethyl Orthosilicate in Acidic Condition. Journal of Sol-Gel Science and Technology 19, 661–664 (2000). https://doi.org/10.1023/A:1008746318901
Issue Date:
DOI: https://doi.org/10.1023/A:1008746318901