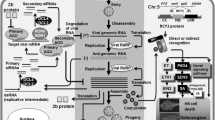
Abstract
Salicylic acid is part of a signal transduction pathway that induces resistance to viruses, bacteria and fungi. In tobacco and Arabidopsis the defensive signal transduction pathway branches downstream of salicylic acid. One branch induces PR-1 proteins and resistance to bacteria and fungi, while the other triggers induction of resistance to RNA and DNA viruses. This virus-specific branch can be activated using antimycin A and cyanide, or inhibited with salicylhydroxamic acid, suggesting a role for alternative oxidase in resistance to viruses. The virus-specific defensive pathway activates multiple resistance mechanisms. In tobacco, salicylic acid induces resistance to systemic movement of cucumber mosaic virus but has no effect on its replication or cell-to-cell movement. However, in the case of tobacco mosaic virus in tobacco, salicylic acid appears to induce interference with the synthesis of viral RNA.
Similar content being viewed by others
References
Akad F, Teverovsky E, David A, Czosnek H, Gidoni D, Gera A and Loebenstein G (1999) A cDNA from tobacco codes for an inhibitor of virus replication (IVR)-like protein. Plant Molecular Biology 40: 969-976
Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E and Ryals J (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein-1a. Proceedings of the National Academy of Sciences of the United States of America 90: 7327-7331
Arce-Johnson P, Kahn TW, Reimann-Philipp U, Rivera-Bustamante R and Beachy RN (1995) The amount of movement protein produced in transgenic plants influences the establishment, local movement and systemic spread of infection by movement protein-deficient tobacco mosaic virus. Molecular Plant-Microbe Interactions 8: 415-423
Bachem CWB, van der Hoeven RS, deBruijn SM, Vreugdenhil D, Zabeau M and Visser RGF (1996) Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: Analysis of gene expression during potato tuber development. Plant Journal 9: 745-753
Baker B, Zambryski P, Staskawicz B and Dinesh-Kumar SP (1997) Signalling in plant-microbe interactions. Science 276: 726-733
Baulcombe DC (1999) Fast forward genetics based on virusinduced gene silencing. Current Opinion in Plant Biology 2: 109-113
Cao H, Glazebrook J, Clarke JD, Volko S and Dong XN (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57-63
Carson RAJ (1999) PhD Thesis, University of Cambridge, UK
Chivasa S, Berry O, apRees T and Carr JP (1999) Changes in gene expression during development and thermogenesis in Arum. Australian Journal of Plant Physiology 26: 391-399
Chivasa S and Carr JP (1998) Cyanide restores N genemediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell 10: 1489-1498
Chivasa S, Murphy AM, Naylor M and Carr JP (1997) Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell 9: 547-557
Cutt JR, Harpster MH, Dixon DC, Carr JP, Dunsmuir P and Klessig DF (1989) Disease response to tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology 173: 89-97
Dangl JL, Dietrich RA and Richberg MH (1996) Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 8: 1793-1807
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E and Ryals J (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247-1250
Dempsey DA, Shah J and Klessig DF (1999) Salicylic acid and disease resistance in plants. Critical Reviews in Plant Science 18: 547-575
Després C, DeLong C, Glaze S, Liu E and Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279-290
Draper J (1997) Salicylate, superoxide synthesis, and cell suicide in plant defence. Trends in Plant Sciences 2: 162-165
Durrant WE, Rowland O, Piedras P, Hammond-Kosack KE and Jones JDG (2000) cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12: 963-977
Fraser RSS and Clay CM (1983) Pathogenesis-related proteins and acquired resistance - causal relationship or separate effects? Netherlands Journal of Plant Pathology 89: 283-292
GenoudTand Métraux J P (1999) Crosstalk in plant cell signaling: structure and function of the genetic network. Trends in Plant Science 4: 503-507
Gianinazzi S, Martin C and Vallée J-C (1970) Hypersensibilité aux virus, température et protéines solubles chez le Nicotiana ‘Xanthi nc’ Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. CR Acad Sci Paris D 270: 2383-2386
Hammerschmidt R (1999a) Induced disease resistance: how do induced plants stop pathogens? Physiological and Molecular Plant Pathology 55: 77-84
Hammerschmidt R (1999b) Phytoalexins: What have we learned after 60 years? Annual Review of Phytopathology 37: 285-306
Hooft van Huijsduijnen RAMH, Alblas SW, DeRijk RH and Bol JF (1986) Induction by salicylic acid of pathogenesisrelated proteins and resistance to alfalfa mosaic virus infection in various plant species. Journal of General Virology 67: 2135-2143
Kachroo P, Yoshioka K, Shah J, Dooner HK and Klessig DF (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677-690
Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S and Ryals J (1994) Induction of systemic acquired disease resistance in plants by chemicals. Annual Review of Phytopathology 32: 439-459
Kuć J (1995) Phytoalexins, stress metabolism, and disease resistance in plants. Annual Reviewof Phytopathology 33: 275-297
Lacomme C and Roby D (1999) Identification of new early markers of the hypersensitive response in Arabidopsis thaliana. FEBS Letters 459: 149-153
Laties GG (1982) The cyanide-resistant, alternative path in higher plant respiration. Annual Reviewof Plant Physiology and Plant Molecular Biology 33: 519-555
Lennon AM, Neuenschwander UH, RibasCarbo M, Giles L, Ryals JA and Siedow JN (1997) The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiology 115: 783-791
Linthorst HJM, Meuwissen RLJ, Kauffmann S and Bol JF (1989) Constitutive expression of pathogenesis-related proteins PR-1, GRP, and PR-S in tobacco has no effect on virus infection. Plant Cell 1: 285-291
Loebenstein G and Gera A (1981) Inhibitor of virus replication released from tobacco mosaic virus-infected protoplasts of a local lesion-responding tobacco cultivar. Virology 114: 132-139
Malamy J, Carr JP, Klessig DF and Raskin I (1990) Salicylic acid - a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002-1004
Maxwell DP, Wang Y and McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences of the United States of America 96: 8271-8276
McDowell JM and Dangl JL (2000) Signal transduction in the plant immune response. Trends in Biochemical Sciences 25: 79-82
Meeuse BJD and Raskin I (1988) Sexual reproduction in the Arum lily family, with emphasis on thermogenicity. Sexual Reproduction 1: 3-15
Métraux JP, Signer H, Ryals J, Ward E, Wyssbenz M, Gaudin J, Raschdorf K, Schmid E, Blum W and Inverardi B (1990) Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250: 1004-1006
Mittler R, Shulaev V, Seskar M and Lam E (1996) Inhibition of programmed cell death in tobacco plants during a pathogeninduced hypersensitive response at low oxygen pressure. Plant Cell 8: 1991-2001
Møller IA, Bérczi A, van der Plas LHW and Lambers H (1988) Measurement of the activity and capacity of the alternative oxidase in intact plant tissues: Identification of problems and possible solutions. Physiologia Plantarum 72: 642-649
Moller SG and Chua NH (1999) Interactions and intersections of plant signalling pathways. Journal of Molecular Biology 293: 219-234
Murphy AM, Chivasa S, Singh DP and Carr JP (1999) Salicylic acid-induced resistance to viruses and other pathogens: a parting of the ways? Trends in Plant Science 4: 155-160
Murphy AM, Holcombe LJ and Carr JP (2000) Characteristics of salicylic acid-induced delay in disease caused by a necrotrophic fungal pathogen in tobacco. Physiological and Molecular Plant Pathology 57: 47-54
Naylor M, Murphy AM, Berry JO and Carr JP (1998) Salicylic acid can induce resistance to plant virus movement. Molecular Plant-Microbe Interactions 11: 860-868
Preisig, C L and Kuć JA (1987) Inhibition by salicylhydroxamic acid, BW755C, eicosatetraynoic acid, and disulfiram of hypersensitive resistance elicited by arachidonic-acid or poly-Llysine in potato tuber. Plant Physiology84: 891-894
Raskin I, Ehmann A, Melander WR and Meeuse BJD (1987) Salicylic acid - a natural inducer of heat production in Arum lilies. Science 237: 1601-1602
Rhoads DM and McIntosh L (1993) The salicylic acidinducible alternative oxidase gene AOX1 and genes encoding pathogenesis-related proteins share regions of sequence similarity in their promoters. Plant Molecular Biology 21: 615-624
Ross AF (1961a) Localized acquired resistance to plant virus infection in hypersensitive hosts. Virology 14: 329-339 Ross AF (1961b) Systemic acquired resistance induced by localized virus infection in hypersensitive hosts. 14: 340-358
Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P and Uknes S (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 9: 425-439
Siedow JN and Moore AL (1993) A kinetic model for the regulation of electron transfer through the cyanide-resistant pathway in plant mitochondria. Biochimica et Biophysica Acta 1142: 165-174
Simons BH, Millenaar FF, Mulder L, van Loon LC and Lambers H (1999) Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiology 120: 529-538
Spiegel S, Gera A, Salomon R, Ahl P, Harlap S and Loebenstein G (1989) Recovery of an inhibitor of virus-replication from the intercellular fluid of hypersensitive tobacco infected with tobacco mosaic virus and from uninfected induced-resistant tissue. Phytopathology 79: 258-262
Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG and Jones JDG (1995) Molecular-genetics of plant disease resistance. Science 268: 661-667
Sticher L, MauchMani B and Métraux JP (1997) Systemic acquired resistance. Annual Review of Phytopathology 35: 235-270
Vanlerberghe GC and McIntosh L (1997) Alternative oxidase: From gene to function. Annual Reviewof Plant Physiology and Plant Molecular Biology 48: 703-734
van Loon LC (1983) The induction of pathogenesis-related proteins by pathogens and specific chemicals. Netherlands Journal of Plant Pathology 89: 265-273
van Loon LC and van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. 'samsun’ and 'samsun NN'. II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 40: 199-211
van Loon LC and van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiological and Molecular Plant Pathology 55: 85-97
White RF (1979) Acetylsalicylic acid (aspirin) induces resistance to TMV in tobacco. Virology 99: 410-412
White RF, Antoniw JF, Carr JP and Woods RD (1983) The effects of aspirin and polyacrylic acid on the multiplication and spread of TMV in different cultivars of tobacco with and without the N-gene. Phytopathol Z 107: 224-232
Xie ZX and Chen ZX (1999) Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiology 120: 217-225
Yu IC, Parker J and Bent AF (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proceedings of the National Academy of Sciences of the United States of America 95: 7819-7824
Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J and Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Molecular Plant-Microbe Interactions 13: 191-202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, A.M., Gilliland, A., Eng Wong, C. et al. Signal Transduction in Resistance to Plant Viruses. European Journal of Plant Pathology 107, 121–128 (2001). https://doi.org/10.1023/A:1008732123834
Issue Date:
DOI: https://doi.org/10.1023/A:1008732123834