Abstract
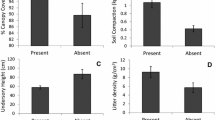
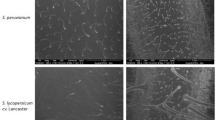
In 1995, conidia of Ulocladium atrum were applied to a canopy of green lily (Lillium spp.) leaves in order to investigate its survival, colonisation of artificially induced necrotic leaf tissues and competitive ability against Botrytis spp. and naturally occurring saprophytes. U. atrum conidia density cm-2 at the top and middle canopy levels was not significantly different following application of the antagonist with a propane powered backpack sprayer. In repeat experiments, conidia density on leaves at the lower canopy level was 18% to 20% of that deposited onto leaves at the top of the lily canopy. There was a significant (P < 0.001) linear decline of U. atrum conidia over time and after 21 days conidia density had declined by up to 73%. Germination of U. atrum on green leaves in the field reached a maximum of 81%, seven days after antagonist application. Conidial viability, measured as germination potential, declined slightly (100% to 88%) after seven days exposure to field conditions but there were no further changes in the germination potential even after 21 days of field exposure. The germination potential was not affected by canopy level. The ability of surviving U. atrum conidia to colonise necrotic tissues, artificially induced with paraquat, was measured. U. atrum colonisation was consistently highest on necrotic leaves at the top level of the canopy and consistently lower on leaves from the bottom canopy level. Necrotic leaf colonisation by U. atrum decreased over time from 51% (necrosis induced immediately after antagonist application) to 21% when necrosis was induced 21 days after antagonist application. A significant (P < 0.001) linear relationship (R2 = 0.713) between colonisation of necrotic tissues and conidia density prior to induction of necrosis was detected. When necrosis was induced immediately after antagonist application, U. atrum outcompeted commonly occurring saprophytic Alternaria spp. and Cladosporium spp. The ability of U. atrum to significantly reduce colonisation by Alternaria spp. was maintained for up to 21 days. Botrytis spp. did not occur in these field experiments. It was concluded that U. atrum had the ability to survive and persist in the phyllosphere for up to 21 days in the field and provided further evidence that U. atrum has the necessary survival characteristics to be a successful biological control agent of Botrytis spp.
Similar content being viewed by others
References
Bannon E (1978) A method of detecting Septoria nodorumon symptomless leaves of wheat. Irish Journal of Agricultural Research 17: 323-324
Begon M, Harper JL and Townsend CR (1986). Ecology: Individuals, Populations and Communities. Blackwell Scientific, Oxford
Bowen JK, Crowhurst RN, Templeton MD and Stewart A (1996) Molecular markers for a Trichoderma harzianumbiological control agent-Introduction of the hygromycin B resistance gene and the Beta-glucuronidase gene by transformation. New Zealand J Crop hort Sci 24: 219-228
Burrage SW (1971). The micro-climate at the leaf surface. In: Preece TF and Dickinson CH (eds) Ecology of Leaf Surface Micro-organisms (pp. 91-101) Academic Press, London Caesar AJ and Pearson RC (1983) Environmental factors affecting survival of ascospores of Sclerotinia sclerotiorum. Phytopathology 73: 1024-1030
Cerkauskas RF and Sinclair JB (1980) Use of Paraquat to aid detection of fungi in soybean tissues. Phytopathology 70: 1036-1038
Chastagner GA and Riley K (1990) Occurrence and control of benzimidazole and dicarboximide resistant Botrytisspp. on bulb crops in Western Washington and Oregon. Acta Hort 266: 437-443
Diem HG (1971) Effect of low humidity on the survival of germinated spores commonly found in the phyllosphere. In: Preece TF and Dickenson CH (eds) Ecology of Leaf Surface Microorganisms (pp. 211-219) Academic Press, London
Doss RP, Chastagner GA and Riley KL (1984) Techniques for inoculum production and inoculation of lily leaves with Botrytis elliptica. Plant Dis 68: 854-856
Elad Y, Zimand G, Zags Y, Zuriel S and Chet I (1993) Use of Trichoderma harzianumin combination or alternation with fungicides to control cucumber grey mould (Botrytiscinerea) under commercial greenhouse conditions. Plant Pathol 42: 324-332
Elmer PAG, Walter M, Köhl J and Boyd-Wilson KSH (1995) Progress towards the biological control of Botrytis cinereain New Zealand kiwifruit orchards. Eur J Plant Pathol-Abstracts of the XIII International Pant Protection Congress, The Hague, The Netherlands, July 1995, Abstract 1415
Fitt BDL, McCartney HA and Walklate PJ (1989) Role of rain in dispersal of pathogen inoculum. Annu Rev Phytopathol 27: 241- 270
Fokkema NJ (1993) Opportunities and problems of control of foliar pathogens with micro-organisms. Pestic Sci 37: 411-416
Gullino ML (1992) Chemical control of Botrytisspp. In: Verhoeff K, Malathrakis NE Williamson B (eds) Recent Advances in BotrytisResearch (pp. 217-222) Pudoc Scientific, Wageningen
Heye CC and Andrews JH (1983) Antagonism of Athelia bombacinaand Chaetomium globosumto the apple scab pathogen, Venturia inaequalis. Phytopatholgy 73: 650-654
Jansma JE, van Keulen H and Zadocks JC (1993) Crop protection in the year 2000: a comparison of current policies towards agroejpp864. chemical usage in four West European countries. Crop Prot 12: 483-489
Köhl J, Bélanger RR and Fokkema NJ (1997) Interaction of four antagonistic fungi with Botrytis acladain dead onion leaves: A comparative microscopic and ultrastructural study. Phytopathology 87: 634-642
Köhl J, Molhoek WML, van der Plas CH and Fokkema NJ (1995a) Suppression of sporulation of Botrytisspp. as a valid biocontrol strategy. Eur J Plant Pathol 101: 251-259
Köhl J, Molhoek WML, van der Plas CH and Fokkema NJ (1995b). Effect of Ulocladium atrumand other antagonists on sporulation of Botrytis cinereaon dead lily leaves exposed to field conditions. Phytopathology 85: 393-401
Köhl J and Schlösser E (1989) Decay of sclerotia of Botrytis cinereaby Trichodermaspp. at low temperatures. J. Phytopathol 125: 320-326
Köhl J, van der Plas CH, Molhoek WML and Fokkema NJ (1995c) Effect of interrupted leaf wetness periods on suppression of sporulation of Botrytis alliiand B. cinereaby antagonists on dead onion leaves. Eur J Plant Pathol 101: 627-637
Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HAS and Harbour A (1995) Antibiotic production and biocontrol activity by Bacillus subtilisCL27 and Bacillus pumilusCL45. J Appl Bacteriol 78: 97-108
Li, H and Leifert C (1994) Development of resistance in Botryotinia fuckeliana(de Bary)Whetzel against the biological control agent Bacillus subtilisCL27. Z Pflanzenkr Pflanzenschutz 101: 414- 418
Michailides TJ and Spotts RA (1991) Effects of certain herbicides on the fate of sporangiospores of Mucor piriformisand conidia of Botrytis cinereaand Penicillium expansum. Pestic Sci 33: 11-22
Migheli Q; Aloi C and Gullino ML (1990) Resistance of Botrytis ellipticato fungicides. Acta Hort 266: 429-436
Nair NG and Allen RN (1993) Infection of grape flowers and berries by Botrytis cinereaas a function of time and temperature. Mycol Res 97: 1012-1014
Pfender WF, Zhang W and Nus A (1993) Biological control to reduce inoculum of the tan spot pathogen Pyrenophora triticirepentisin surface-borne residues of wheat fields. Phytopathology 83: 371-375
Redmond JC, Marois JJ and MacDonald JD (1987) Biological control of Botrytis cinereaon roses with epiphytic microorganisms. Plant Dis 71: 799-802
Rotem J, Wooding B and Aylor DE (1985) The role of solar radiation, especially ultraviolet, in the mortality of fungal spores. Phytopathology 75: 510-514
Swadling IR and Jeffries P (1996) Isolation of microbial antagonists for biocontrol of grey mould diseases of strawberries. Biocontrol Sci Technol 6: 125-136
Vloutoglou I, Fitt BDL and Lucas JA 1995. Periodicity and gradients in dispersal of Alternaria linicolain linseed crops. Eur J Plant Pathol 101: 639-653
Wilkinson V and Lucas, R. L. 1969. Influence of herbicides on the competitive ability of fungi to colonise plant tissues. New Phytopathol 68: 701-708
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elmer, P.A., Köhl, J. The survival and saprophytic competitive ability of the Botrytis spp. antagonist Ulocladium atrum in lily canopies. European Journal of Plant Pathology 104, 435–447 (1998). https://doi.org/10.1023/A:1008646418991
Issue Date:
DOI: https://doi.org/10.1023/A:1008646418991