Abstract
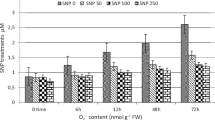
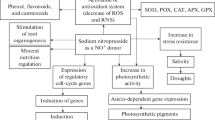
Nitric oxide (NO) is a bioactive molecule involved in many physiological processes. Among its biological function, NO has been proved to be cytotoxic against microorganisms in cells of the immune response, thus preventing infection. We have specifically studied the effect of a NO donor, sodium nitroprusside (SNP), on the chlorophyll content in potato leaves infected with the pathogenic fungus Phytophthora infestans (Pi). Fifteen days after infection, chlorophyll content strongly decayed in water-treated potato leaf sections. SNP was able to partially revert that loss in a dose-dependent manner, being the effective SNP concentrations between 10 µM and 100 µM. NaNO2 and NaNO3, the SNP-derived residual products, were unable to prevent the chlorophyll loss. Treatments with SNP did not affect the survival of Pi and the fungus was able to grow in a V8-agar medium containing 100 µM SNP. Both the amount and the extent of germination of Pi sporangia resulted similar in the absence and in the presence of SNP. Respiratory inhibitors of the cyanide-sensitive and cyanide-resistant pathways, 2,4-dinitrophenol and salicylhydroxamic acid respectively, did not change the chlorophyll levels in infected potato leaves, suggesting that NO effect should not be on mitochondrial respiration. These results indicate that NO could be a protective molecule, either preserving the chloroplast membrane of infected leaf sections against the toxicity of reactive oxygen species or being directly involved in any step of the chlorophyll metabolic pathway.
Similar content being viewed by others
References
Anbar M (1995) Nitric oxide: A synchronizing chemical messenger. Experientia 51: 545–550
Arnon, DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15
Baker CJ and Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33: 299–321
Borutait´e V and Brown GC (1996) Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mi tochondrial respiration by nitric oxide. Biochem J 315: 295–299
Bowler C, Neuhaus G, Yamagata H and Chua NH (1994) Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 77: 73–81
Croft KP, Voisey CR and Slusarenko AJ (1990) Mechanisms of hypersensitive cell collapse: Correlation of increased lipoxygenase activity with membrane damage in leaves of Phaseolus vulgaris (L.) cv. Red Mexican inoculated with avirulent race 1 cells of Pseudomonas syringae pv. phaseolicola. Physiol & Mol Plant Pathol 36: 49–62
DoKe N and Tomiyama K (1977) Effect of high molecular weight substances released from zoospores of Phytophthora infestans in the hypersensitive response of potato tuber. Phytopathol Z 90: 236–242
Drapier JC and Hibbs JB (1988) Differentiation of murine macrophage to express non-specific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfer enzymes in the macrophage effector cells. J Immunol 140: 3829–3838
Freytag S, Arabatziz N, Hahlbrock K and Schmelzer E (1994) Reversible cytoplasmic rearrangements precede wall apposition, hypersensitive cell death and defense-related gene activation in potato-Phytophthora infestans interactions. Planta 194: 123–135.
Fritzemeier KH, Cretin C, Kombrink E, Rohwer F, Taylor J, Scheel D and Hahlbrock K (1987) Transient induction of phenylalanine ammonia-layse and 4-coumarate:CoA ligase mRNAs in potato leaves infected with virulent or avirulent races of Phytophthora infestans. Plant Physiol 85: 34–41
Goodman RN (1972) Electrolyte leakage and membrane damage in relation to bacterial population, pH and ammonia production in tobacco leaf tissue inoculated with Pseudomonas pisi. Phytopathol 62: 1327–1331
Goodman RN and Novacky AJ (1994) The Hypersensitive Reaction in Plants to pathogens. A resistance phenomenon (p 16) APS Press. The American Phytopathological Society St. Paul, Minnesota, USA
Hammond-Kosack KE and Jones JDG (1996) Resistance gene-dependent plant defense responses. The Plant Cell 8: 1773–1791
Hibbs JB, Taintor Jr, RR, Vavrin Z and Rachlin EM (1988) Nitric oxide: A cytotoxic activated macrophage effectors molecule. Biochem Biophys Res Commun 157: 87–94
Ignarro LJ (1990) Biosynthesis and metabolism of endothelium-derivated nitric oxide. A Rev Pharmacol Toxicol 30: 535–560
Khan AU and Wilson T (1995) Reactive oxygen species as cellular messengers. Chemistry and Biology 2: 437–445
Kr¨oncke KD and Kolb-Bachofen V (1996) Detection of Nitric Oxide interaction with zinc finger proteins. In: Packer L (ed) Methods in Enzymology, Vol 269 (pp 279–284) Academic Press, Inc, San Diego
Kunert J (1995) Effect of nitric oxide donors on survival of conidia, germination and growth of Aspergillus fumigatus in vitro. Folia Microbiol 40(3): 238–244
Larkman AU and Jack JJB (1995) Synaptic plasticity: Hippocampal LTP. Curr Opin Neurobiol 5: 524–534
Laxalt AM, Cassia RO, Sanllorenti PM, Madrid EA, Andreu AB, Daleo GR, Conde RD and Lamattina L (1996) Accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase RNA under biological stress conditions and elicitor treatments in potato. Plant Mol Biol 30: 961–972
Lepoivre M, Fieschi J, Coves J, Thelander L and Fontecave (1991) Inactivation of ribonucleotide reductase by nitric oxide. M Biochem Biophys Res Commun 179: 442–448
Leshesm YY and Haramaty E (1996) Plant aging: The emission of NO and ethylene and effect of NO-releasing compounds on growth of pea (Pisum sativum) foliage. J Plant Physiol 148: 258–263
Leshesm YY (1996) Nitric Oxide in biological systems. Plant Growth Regulation 18: 155–159
Marletta MA and Tayeh, MA (1993) Nitric oxide synthase structure and mechanism. J Biol Chem 268: 12231–12234
Miller MJS, Thompson JH, Liu X, Eloby-Childress S, Sadowska-Krowicka H, Jing Zhang X and Clark DA (1996). Failure of L-NAME to cause inhibition of nitric oxide synthesis: Role of inducible nitric oxide synthase. Inflamm Res 45: 272–276
Modolell M, Eichmann K and Soler G (1997) Oxidation of N G-hydroxyl-L-arginine to nitric oxide mediated by respiratory burst: An alternative pathway to NO synthesis. FEBS Lett 401: 123–126
Moilanen E and Vapaatalo H (1995) Nitric oxide in inflammation and immune response. Annals of Medicine 27: 359–367
Moncada SM, Palmer RMJ and Higgs EA (1991) Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142
Neuhaus G, Bowler C, Kern R and Chua NH (1993) Calcium/ calmodulin-dependent and-independent phytochrome signal transduction pathways. Cell 73: 937–952.
Noack E and Feelisch M (1991) Molecular mechanisms of nitrova-sodilator bioactivation. Basic Res Cardiol 86 (suppl 2): 37–50
Noritake T, Kawakita K and Doke N (1996) Nitric oxide induces phytoalexin accumulation in potato tuber tissues. Plant Cell Physiol 37: 113–116
Nozue M, Tomiyama K and Doke N (1978) Effect of adenosine 50-triphosphate on hypersensitive death of potato tuber cells infected by Phytophthora infestans. Phytopathology 68: 873–876
Pfeiffer S, Jankstin B, Soja G, Koesling D, Mayer B and Eberman R (1995) Detection of nitric oxide-sensitive guanylil cyclase in higher plants. J Endothelial Cell Res, Vol 3. Abstract 66
Poderoso JJ, Carreras MC, Lisdero C, Riob´o N, Sch¨opfer F and Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328: 85–92
Satler SO and Thimann KV (1983) Relation between respiration and senescence in Oat leaves. Plant Physiol 72: 540–546
Schmidt HHHW, Pollock JS, Nakane M, Gorsky LD, Forstermann U and Murad F (1991) Purification of a soluble isoform of guanylil cyclase-activating-factor synthase. Proc Natl Acad Sci USA 88: 365–369
Schmidt HHHW, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD and Feelisch (1996) No●NO from NO synthase. Proc Natl Acad Sci USA 93: 14492–14497
Schr¨oder M, Hahlbrock K and Kombrink E (1992) Temporal and spatial patterns of 1,3-β-glucanase and chitinase induction in potato leaves infected by Phytophthora infestans. Plant J2: 161–172
Sen S and Cheema IR (1995) Nitric oxide synthase and calmodulin immunoreactivity in plant embryonic tissue. Biochem Arch 11: 221–227
Snyder SH (1992) Nitric oxide: First in a new class of neurotransmitters? Science 257: 494
Stamler JS, Singel DJ and Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902
Stamler JS (1994) Redox signaling: Nitrosylation and related targets interactions of nitric oxide. Cell 78: 931–936
Stintzi A, Heitz T, Prasad V, Wiedemann-Medinoglu S, Kauffmann S, Geoffroy P, Legrand M and Fritig B (1993) Plant pathogenesis-related proteins and their role in defense against pathogens. Biochemie 75: 687–706
Sutherland MW (1991) The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol 39: 79–94
Takehara Y, Nakahara H, Inai Y, Yabuki M, Yoshioka T, Inove M, Horton AA and Utsumik (1996) Oxygen-dependent reversible inhibition of mitochondrial respiration by nitric oxide. Cell Structure and Function 21: 251–258
Weinberg DE (1992) Iron depletion: A defense against intracellular infection and neoplasm. Lif Sci 50: 1289–1297
Author information
Authors and Affiliations
Additional information
(both authors contributed equally to this work)
Rights and permissions
About this article
Cite this article
Lazalt, A., Beligni, M. & Lamattina*, L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. European Journal of Plant Pathology 103, 643–651 (1997). https://doi.org/10.1023/A:1008604410875
Issue Date:
DOI: https://doi.org/10.1023/A:1008604410875