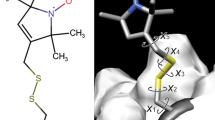
Abstract
NMR investigations of larger macromolecules (>20 kDa) are severely hindered by rapid 1H and 13C transverse relaxation. Replacement of non-exchangeable protons with deuterium removes many efficient 1H-1H and 1H-13C relaxation pathways. The main disadvantage of deuteration is that many of the protons which would normally be the source of NOE-based distance restraints are removed. We report the development of a novel labeling strategy which is based on specific protonation and 14N-labeling of the residues phenylalanine, tyrosine, threonine, isoleucine and valine in a fully deuterated, 15N-labeled background. This allows the application of heteronuclear half-filters, 15N-editing and 1H-TOCSY experiments to select for particular magnetization transfer pathways. Results from investigations of a 47 kDa dimeric protein labeled in this way demonstrated that the method provides useful information for the structure determination of large proteins.
Similar content being viewed by others
References
Arrowsmith, C.H., Pachter, R., Altman, R.B., Iyer, S.B. and Jardetzky, O. (1990) Biochemistry, 29, 6332–6341.
Chou, P.Y. and Fasman, G.D. (1974) Biochemistry, 13, 211–222.
Clore, G.M. and Gronenborn, A.M. (1994) Methods Enzymol., 239, 349–363.
Englander, S.W. and Wand, A.J. (1987) Biochemistry, 26, 5953–5958.
Gardner, K.H. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 7599–7600.
Gardner, K.H., Rosen, M.K. and Kay, L.E. (1997) Biochemistry, 36, 1389–1401.
Grzesiek, S., Doebeli, H., Gentz, R., Garotta, G., Labhardt, A.M. and Bax, A. (1992) Biochemistry, 31, 8180–8190.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993) J. Am. Chem. Soc., 115, 4369–4370.
Grzesiek, S. and Bax, A. (1993) J. Biomol. NMR, 3, 185–204.
Kay, L.E. and Gardner, K.H. (1997) Curr. Opin. Struct. Biol., 7, 722–731.
LeMaster, D.M. (1990) Quart. Rev. Biophys., 23, 133–174.
Maniatis, T., Fritsch, E.F. and Sambrook, J. (1982) Molecular Cloning. A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Metzler, W.J., Wittekind, M., Goldfarb, V., Mueller, L. and Farmer II, B.T. (1996) J. Am. Chem. Soc., 118, 6800–6801.
Minor, D.L. and Kim, P.S. (1994) Nature, 367, 660–663.
Nietlispach, D., Clowes, R.T., Broadhurst, R.W., Ito, Y., Keeler, J., Kelly, M., Ashurst, J., Oschkinat, H., Domaille, P.J. and Laue, E.D. (1996) J. Am. Chem. Soc., 118, 407–415.
Otting, G. and Wüthrich, K. (1990) Quart. Rev. Biophys., 23, 39–96.
Pittard, A.J. (1987) In Escherichia coli and Salmonella Typhimurium (Ed., Neidhardt, F.C.), Vol. 1, American Society for Microbiology, Washington, DC, pp. 368–394.
Reitzer, L.J. and Magasanik, B. (1987) In Escherichia coli and Salmonella Typhimurium (Ed., Neidhardt, F.C.), Vol. 1, American Society for Microbiology, Washington, DC, pp. 302–320.
Richter, G., Volk, R., Krieger, C., Lahm, H.-W., Röthlisberger, U. and Bacher, A. (1992) J. Bacteriol., 174, 4050–4056.
Richter, G., Kelly, M.J.S., Krieger, C., Yu, Y., Bermel, W., Karlsson, G., Bacher, A. and Oschkinat, H. (1999) Eur. J. Biochem., 261, 57–65.
Rosen, M.K., Gardner, K.H., Willis, R.C., Parris, W.E., Pawson, T. and Kay, L.E. (1996) J. Mol. Biol., 263, 627–636.
Shan, X., Gardner, K.H., Muhandiram, D.R., Rao, N.S. and Kay, L.E. (1996) J. Am. Chem. Soc., 118, 6570–6579.
Smith, B.O., Ito, Y., Raine, A., Teichmann, S., Ben-Tovim, L., Nietlispach, D., Broadhurst, R.W., Terada, T., Kelly, M., Oschkinat, H., Shibata, T., Yokoyama, S. and Laue, E.D. (1996) J. Biomol. NMR, 8, 360–368.
Smith, C.K., Withka, J.M. and Regan, L. (1994) Biochemistry, 33, 5510–5517.
Torchia, D.A., Sparks, S.W. and Bax, A. (1988a) Biochemistry, 27, 5135–5141.
Torchia, D.A., Sparks, S.W. and Bax, A. (1988b) J. Am. Chem. Soc., 110, 2320–2321.
Umbarger, H.E. (1987) In Escherichia coli and Salmonella Typhimurium (Ed., Neidhardt, F.C.), Vol. 1, American Society for Microbiology, Washington, DC, pp. 352–367.
Vuister, G.W., Kim, S.-J. and Bax, A. (1994) J. Am. Chem. Soc., 116, 9206–9210.
Wagner, G. (1993) J. Biomol. NMR, 3, 375–385.
Yamazaki, T., Lee, W., Arrowsmith, C.H., Muhandiram, D.R. and Kay, L.E. (1994a) J. Am. Chem. Soc., 116, 11655–11666.
Yamazaki, T., Lee, W., Revington, M., Mattiello, D.L., Dahlquist, F.W., Arrowsmith, C.H., Muhandiram, D.R. and Kay, L.E. (1994b) J. Am. Chem. Soc., 116, 6464–6465.
Zhang, H., Zhao, D., Revington, M., Lee, W., Jia, X., Arrowsmith, C.H. and Jardetzky, O. (1994) J. Mol. Biol., 238, 592–614.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelly, M.J., Krieger, C., Ball, L.J. et al. Application of amino acid type-specific 1H- and 14N-labeling in a 2H-, 15N-labeled background to a 47 kDa homodimer: Potential for NMR structure determination of large proteins. J Biomol NMR 14, 79–83 (1999). https://doi.org/10.1023/A:1008351606073
Issue Date:
DOI: https://doi.org/10.1023/A:1008351606073