Abstract
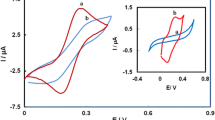
The redox properties of ubiquinone 10 (UQ10) placed at the air–water interface were studied using the horizontal touching method with a thin mercury film (TMFE) working electrode and cyclic voltammetry. Changes of pH of the subphase affected the formal potential of the ubiquinone/ubiquinol system exhibiting the participation of protons in the overall reduction of UQ10. The protonation of the semiquinone transition product was found to be the rate determining step. This explains the dependence of the rate constant value on pH. The highest values of rate constants were found at pH over 13. Under these conditions the mechanism of the process is different. The concentration of protons is small, and the availability of the counter ions (i.e., K+) becomes crucial for the kinetics of reduction. Their role is to neutralize the negative charge of the redox group following its reduction. The logarithm of rate constants was found to decrease linearly with the increase of surface concentration of ubiquinone. This reflects the influence of intermolecular interactions in the monolayer on the kinetics of the electrode process.
Similar content being viewed by others
References
B. Chazotte and C. R. Hackenbrock: J. Biol. Chem. 264, 4978 (1989).
S. E. Boesch and R. A. Wheeler: J. Phys. Chem. A 101, 5799 (1997).
F. L. O'Brien and J. W. Oliver: Anal. Chem. 41, 1810 (1969).
L. E. Morrison, J. E. Schelhorn, T. M. Cotton, C. L. Bering, and P. A. Loach: in B. L. Trumpower (ed.), Function of Quinones in Energy Conserving Systems, Academic Press, New York (1982), p. 35.
K. Takamura, A. Mori, and F. Kusu: Bioelectrochem. Bioenerg. 9, 499 (1982).
O. S. Ksenzhek, S. A. Petrova, and M. V. Kolodyazhny: Bioelectrochem. Bioenerg. 9, 167 (1982).
R. S. Schrebler, A. Arratia, S. Sanchez, M. Haun, and N. Duran: Bioelectrochem. Bioenerg. 23, 81 (1990).
K. Takehara and Y. Ide: Bioelectrochem. Bioenerg. 26, 297 (1991).
R. Bilewicz: Polish J. Chem. 67, 1695 (1993).
J. M. Laval and M. Majda: Thin Solid Films 244, 836 (1994).
K. Takehara, H. Takemura, Y. Ide, and S. Okayama: J. Electroanal. Chem. 308, 345 (1991).
Z. Stojek and Z. Kublik: J. Electroanal. Chem. 60, 349 (1975).
M. Donten and Z. Kublik: J. Electroanal. Chem. 196, 275 (1985).
I. Langmuir and V. Schaefer: J. Am. Chem. Soc. 60, 1351 (1938).
M. Fujihira and T. Araki: Chem. Lett. 921 (1986).
X. Zhang and A. J. Bard: J. Am. Chem. Soc. 111, 8098 (1989).
K. Odashima, M. Kotato, M. Sugawara, and Y. Umezawa: Anal. Chem. 65, 927 (1993).
H. Daifuku, K. Aoki, K. Tokuda, and H. Matsuda: J. Electroanal. Chem. 183, 1 (1985).
G. J. Gordillo and D. J. Schiffrin: J. Chem. Soc. Faraday Trans. 90, 1913 (1994).
E. Laviron: J. Electroanal. Chem. 101, 19 (1979).
M. R. Moncelli, L. Becucci, A. Nelson, and R. Guidelli: Biophys. J. 70, 2716 (1996).
E. Laviron: J. Electroanal. Chem. 52, 395 (1974).
A. P. Brown and F. C. Anson: Anal. Chem. 49, 1589 (1977).
C. E. D. Chidsey, C. R. Bertozzi, T. M. Putvinski, and A. M. Mujsce: J. Am. Chem. Soc. 112, 4301 (1990).
S. E. Creager and G. K. Rowe: Anal. Chim. Acta 246, 233 (1991).
T. Nagaoka, N. Nishii, K. Fujii, and K. Ogura: J. Electranal. Chem. 322, 383 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sek, S., Bilewicz, R. Voltammetric Probing of Molecular Assemblies of Ubiquinone-10 at the Air–Water Interfaces. Journal of Inclusion Phenomena 35, 55–62 (1999). https://doi.org/10.1023/A:1008194314304
Issue Date:
DOI: https://doi.org/10.1023/A:1008194314304