Abstract
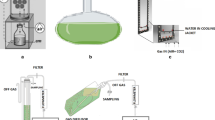
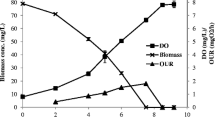
The growth characteristics and nutrient removal fromsynthetic wastewater by Rhodobacter sphaeroides,Chlorella sorokiniana and Spirulinaplatensis were investigated under aerobic dark(heterotrophic) and aerobic light (photoheterotrophic)conditions. Both in terms of economy and efficiency,aerobic dark conditions were the best for wastewatertreatment using R. sphaeroides and C.sorokiniana, but light was necessary with S.platensis. Neither growth nor nutrient removalcharacteristics of the cells were affected insynthetic wastewater with as high as 10 000 ppmacetate, 1000 ppm propionate, 700 ppm nitrate and 100 ppmphosphate. Although R. sphaeroides and C. sorokiniana showed good growth in syntheticwastewater containing 400 ppm of ammonia, S.platensis was completely inhibited.When grown as a monoculture, none of thestrains could simultaneously remove acetate,propionate, ammonia, nitrate and phosphate from thewastewater. R. sphaeroides could remove allthe above nutrients except nitrate, but the rate of removal was relatively low. The rate of nutrientsremoval by C. sorokiniana was higher, but theorganism could not remove propionate; S.platensis could efficiently remove nitrate, ammoniaand phosphate, but none of the organic acids. A mixedculture of R. sphaeroides and C.sorokiniana was therefore used for simultaneousremoval of organic acids, nitrate, ammonia andphosphate. The optimum ratio of the cells depended onthe composition of the wastewater.
Similar content being viewed by others
References
Aharon A, Yosef A (1976) Toxicity of ammonia to algae in sewage oxidation ponds. Appl. envir. Microbiol. 31: 801-806.
Boussiba S, Vonshak A, Cohen Z, Avissar Y, Richmond A (1987) Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 12: 37-47.
Dor I (1975) High density, dialysis culture of algae on sewage. Wat. Res. 9: 251-254.
Doran MD, Boyle W (1979) Phosphorus removal by activated algae. Wat. Res. 13: 805-812.
Gantar M, Obreht Z, Dalmacija B (1991) Nutrient removal and algae succession during the growth of Spirulina platensis and Scenedesmus quadricauda on swine wastewater. Biores. Technol. 36: 167-171.
Glombitza KW, Koh M (1989) Secondary metabolites of pharmaceutical potentials. In Cresswell RC, Rees TAV, Shah N (eds), Algal and Cyanobacterial Biotechnology, Longman, Harlow, 161-238.
Hashimoto S, Furukawa K (1989) Nutrient removal from secondary effluent by filamentous algae. J. Ferment. Bioengng 67: 62-69.
Kishimoto M, Okakura T, Nagashima H, Minowa T, Yakayama S, Yamaberi K (1994) CO2 fixation and oil production using microalgae. J. Ferment. Bioengng 78: 479-482.
Negoro M, Shioji N, Miyamoto K, Miura Y (1991) Growth of microalgae in high CO2 gas and effects of SOX and NOX. Appl. Biochem. Biotech. 28/29: 877-886.
Ogbonna JC, Masui H, Tanaka H (1997) Sequential heterotrophic/autotrophic cultivation-An efficient method of producing Chlorella biomass for health food and animal feed. J. appl. Phycol. 9: 359-366.
Ogbonna JC, Tanaka H (1996) Night biomass loss and changes in biochemical composition of cells during light/dark cyclic culture of Chlorella pyrenoidosa. J. Ferment. Bioengng 82: 558-564.
Ogbonna JC, Yada H, Tanaka H (1995) Effect of cell movement by random mixing between the surface and bottom of photobioreactors on algal productivity. J. Ferment. Bioengng 79: 152-157.
Sasaki K, Mori H, Nishizawa Y, Nagai S (1988) Denitrifying and photoheterotrophic growth of Rhodobacter sphaeroides S under anaerobic-dark and light conditions. J. Ferment. Technol. 66: 27-32.
Sawayama S, Inoue S, Yokoyama S (1994) Continuous culture of hydrocarbon-rich microalga Botryococcus braunii in secondarily treated sewage. Appl. Microbiol. Biotechnol. 41: 729-731.
Shelef G, Oswald WJ, Golueke CG (1969) The continuous culture of algal biomass on wastes. In Malek I (ed.), Continuous Cultivation of Microorganisms. Prague Academy, 601-629.
Shen J, Hirayama O (1991) Hydrogen photoproduction and denitrification by photosynthetic bacteria isolated from Lake Nakaumi and its vicinity. J. Ferment. Bioengng 72: 338-342.
Travieso L, Benitez F, Weiland P, Sãnchez E, Dupeyrõn R, Dominguez AR (1996) Experiments on immobilization of microalgae for nutrient removal in wastewater treatments. Biores. Technol. 55: 181-186.
Yoshihara K, Nagase H, Eguchi K, Hirata K, Miyamoto K (1996) Biological elimination of nitric oxide and carbon dioxide from flue gas by marine microalga NOA-113 cultivated in long tubular photobioreactor. J. Ferment. Bioengng 82: 351-354.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ogbonna, J.C., Yoshizawa, H. & Tanaka, H. Treatment of high strength organic wastewater by a mixed culture of photosynthetic microorganisms. Journal of Applied Phycology 12, 277–284 (2000). https://doi.org/10.1023/A:1008188311681
Issue Date:
DOI: https://doi.org/10.1023/A:1008188311681