Abstract
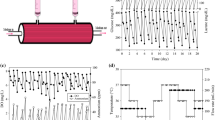
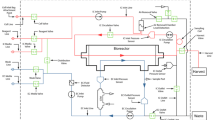
In this article, cell growth in a novel micro hollow fiberbioreactor was compared to that in a T-flask and theAcuSyst-Maximizer®, a large scale industrial hollowfiber bioreactor system. In T-flasks, there was relativelylittle difference in the growth rates of one murine hybridomacultured in three different media and for three other murinehybridomas cultured in one medium. However, substantialdifferences were seen in the growth rates of cells in themicro bioreactor under these same conditions. These differencecorrelated well with the corresponding rates of initial cellexpansion in the Maximizer. Quantitative prediction of thesteady-state antibody production rate in the Maximizer was moreproblematic. However, conditions which lead to faster initialcell growth and higher viable cell densities in the microbioreactor correlated with better performance of a cell line inthe Maximizer. These results demonstrate that the microbioreactor is more useful than a T-flask for determining optimalconditions for cell growth in a large scale hollow fiberbioreactor system.
Similar content being viewed by others
References
Ala-Uotila S, Marjamaki A, Matikainen M-T and Jalkanen M (1994) Use of a hollow fiber bioreactor for large scale production of α2-adrenoceptors in mammalian cells. J Biotechnol 37: 179–184.
Banik GG and Heath CA (1996) High density hybridoma perfusion culture: Limitation vs. inhibition. Appl Biochem Biotechnol 61: 211–229.
Brotherton JD and Chau PC (1996) Modeling of axial flow hollow fiber cell culture bioreactors. Biotechnol Prog 12: 575–590.
Chuppa S, Tsai Y-S, Yoon S, Shakelford S, Rozales C, Bhat R, Tsay G, Matanguihan C, Konstantinov K and Naveh D (1997) Fermentor temperature as a tool for control of high density perfusion cultures of mammalian cells. Biotechnol Bioeng 55: 328–338.
Gramer MJ, Poeschl DM, Conroy MJ and Hammer BE (1999) Effect of harvesting protocol on performance of a hollow fiber bioreactor. Biotechnol Bioeng 65: 334–340.
Gramer MJ and Poeschl DM (1998) Screening tool for hollow fiber bioreactor process development. Biotechnol Prog 14: 203–209.
Hirschel MD and Gruenberg ML (1988) An automated hollow fiber system for the large scale manufacture of mammalian cell secreted product. In: BK Lydersen (ed) Large Scale Cell Culture Technology. Macmillan, pp. 113–144.
Kidwell WR (1989) Filtering out inhibition. Bio/Technology 7: 462–463.
Knazek RA, Wu Y-W, Aebersold PM and Rosenberg SA (1990) Culture of human tumor infiltrating lymphocytes in hollow fiber bioreactors. J Immunol Methods 127: 29–37.
Liu JJ, Chen B-S, Tsai T-F, Wu Y-J, Pang VF, Hseih A, Hsieh JH and Chang TH (1991) Long term and large scale cultivation of human hepatoma Hep G2 cells in hollow fiber bioreactor. Cytotechnology 5: 129–139.
Ozturk SS and Palsson BO (1991) Growth, metabolic, and antibody production kinetics of hybridoma cell culture. Biotechnol Prog 7: 481–494.
Ozturk SS, Riley MR and Palsson BO (1992) Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production. Biotechnol Bioeng 39: 418–431.
Palsson BO, Paek S-H, Schwartz RM, Palsson M, Lee G-M, Silver S and Emerson SG (1993) Expansion of human bone marrow progenitor cells in a high density continuous perfusion system. Bio/Technology 11: 368–371.
Patkar AY, Koska J, Taylor DG, Bowen BD and Piret JM(1995) Protein transport in ultrafiltration hollow fiber bioreactors. AIChE J 41: 415–425.
Piret JM and Cooney CL (1991) Model of oxygen transport limitations in hollow fiber bioreactors. Biotechnol Bioeng 37: 80–92.
Ratner PL, Cleary ML and James E (1978) Production of rapid harvest Moloney murine leukemia virus by continuous cell culture on synthetic capillaries. J Virol 26: 536–539.
Richards SM, Garman RD, Keyes L, Kavanagh B and McPherson JM (1998) Prolactin is an antagonist of TGF-β activity and promotes proliferation of murine B cell hybridomas. Cell Immunol 184: 85–91.
Schlapfer BS, Scheibler M, Holtorf A-P, Nguyen HV and Pluschke G (1995) Development of optimized transfectoma cell lines for production of chimeric antibodies in hollow fiber cell culture systems. Biotechnol Bioeng 45: 310–319.
Stronek DF, Hubel A, Shankar RA, Burger SR, Pan D, McCullough J and Whitley CB (1999) Retroviral transduction and expansion of peripheral blood lymphocytes for the treatment of mucopolysaccharidosis type II, Hunter's syndrome. Transfusion 39: 343–350.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gramer, M.J., Poeschl, D.M. Comparison of cell growth in T-flasks, in micro hollow fiber bioreactors, and in an industrial scale hollow fiber bioreactor system. Cytotechnology 34, 111–119 (2000). https://doi.org/10.1023/A:1008167713696
Issue Date:
DOI: https://doi.org/10.1023/A:1008167713696