Abstract
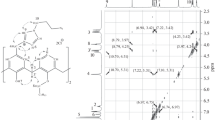
Consecutive base-catalysed reactions of calix[4]arene with the ditosylates of two different polyetherdiols result in the relatively efficient production of an unsymmetrical calix biscrown-6 which, after reduction of an ethoxycarbonyl substituent to an hydroxymethyl group, has been characterised structurally by a room-temperature, single crystal X-ray study of its chloroform solvate. Crystals of ( 4), C53H62O13... ∼0.57CHCl3, are triclinic, P¯1, a 18.95(3), b 12.394(9), c 11.756(7) Å α 106.93(5), β 101.77(8), γ 94.63(8)°, Z = 2; R was 0.088 for 2231 ’observed' (I > 3σ(I)) diffractometer data.
Similar content being viewed by others
References
Z. Asfari, S. Wenger, and J. Vicens: in J. Vicens, Z. Asfari, and J.M. Harrowfield (eds.), Calixarenes’ 50th Anniversary, Kluwer Academic Publishers, Dordrecht (1995), p. 137. (Also published as J. Incl. Phenom. Mol. Recogn. Chem. 19, 137 (1994).) See also Z. Asfari, J-P. Astier, C. Bressot, J. Estienne, G. Pèpe, and J. Vicens: p. 291.
C. Hill, J.-F. Dozol, V. Lamare, H. Rouquette, S. Eymard, B. Tournois, J. Vicens, Z. Asfari, C. Bressot, R. Ungaro, and A. Casnati: in J. Vicens, Z. Asfari, and J.M. Harrowfield (eds.), Calixarenes’ 50th Anniversary, Kluwer Academic Publishers, Dordrecht, 1995, p. 399. (Also published as J. Incl. Phenom. Mol. Recogn. Chem. 19, 399 (1994).)
S. Wenger, Z. Asfari, and J. Vicens: Pure J. Chem. 67, 1037 (1995).
Z. Asfari, C. Bressot, J. Vicens, C. Hill, J-F. Dozol, H. Rouquette, S. Eymard, V. Lamare, and B. Tournois, Anal. Chem. 67, 3133 (1995).
A. Casnati, A. Pochini, R. Ungaro, F. Ugozzoli, F. Arnaud, S. Fanni, M.-J. Schwing, R.J.M. Egberink, F. De Jong, and D.N. Reinhoudt: J. Am. Chem. Soc. 117, 2767 (1995) and references therein.
L. Cecille, M. Casarici, and L. Pietrelli (eds.): in New Separation Chemistry Techniques for Radioactive Waste and Other Specific Applications, Elsevier Appled Science, London (1991).
R. Ungaro, A. Casnati, F. Ugozzoli, A. Pochini, J.-F. Dozol, C. Hill, and H. Rouquette: Angew. Chem., Int. Ed. Engl. 33, 1506 (1994).
F. Arnaud-Neu, Z. Asfari, B. Souley, and J. Vicens: New J. Chem. 20, 453 (1996).
Z. Asfari, C. Bressot, J.-F. Dozol, V. Lamare, C. Naumann, M. Nierlich, P. ThuÉry, and J. Vicens: New J. Chem. 20, 1183 (1996).
K.N. Koh, K. Araki, S. Shinkai, Z. Asfari, and J. Vicens: Tetrahedron Lett. 36, 6095 (1995).
R. Abidi, Z. Asfari, J.M. Harrowfield, A.N. Sobolev, and J. Vicens: Aust. J.Chem. 49, 183 (1996).
P. ThuÉry, M. Nierlich, C. Bressot, V. Lamare, J.-F. Dozol, Z. Asfari, and J. Vicens, J. Incl. Phenom., Mol. Recogn. Chem. 23, 305 (1995). Structures of the ‘free’ ligands are given by P. ThuÉry, M. Nierlich, Z. Asfari, and J. Vicens, J. Incl. Phenom., Mol. Recogn. Chem. 27, 127 (1997).
R. Ungaro, A. Arduini, A. Casnati, O. Ori, A. Pochini, and F. Ugozzoli: in G. Wipff (ed.), Computational Approaches in Supramolecular Chemistry, NATO ASI Series, Mathematical and Physical Sciences, No. 426, Kluwer Academic Publishers, Dordrecht (1994), p. 277.
D.A. Dougherty, P.C. Kearney, L.S. Mizoue, R.A. Kumpf, J.E. Forman, and A. McCurdy: in G. Wipff (ed.), Computational Approaches in Supramolecular Chemistry, NATO ASI Series, Mathematical and Physical Sciences, No. 426, Kluwer Academic Publishers, Dordrecht (1994), p. 301.
R. Abidi, Z. Asfari, J.M. Harrowfield, C. Naumann, A.N. Sobolev, and J. Vicens: Annales de Quimica 92, 51 (1996).
Z. Asfari, J.M. Harrowfield, A.N. Sobolev, and J. Vicens, Aust. J. Chem. 47, 757 (1994).
R. Abidi, Z. Asfari, J.M. Harrowfield, A.N. Sobolev, and J. Vicens: Aust. J. Chem. 49, 183 (1996).
Z. Asfari, J. Vicens and S. Wenger, Tetrahedron Lett. 35, 8369 (1994).
Z. Asfari, J. Vicens, and S. Wenger: J. Incl. Phenom. Mol. Recognit. Chem. 20, 293 (1995).
B. Pulpoka: Doctoral Thesis, UniversitÉ Louis Pasteur de Strasbourg (1997).
A. Ikeda, T. Tsudera, and S. Shinkai: J. Org. Chem. 62, 3568 (1997).
C.D. Gutsche and M. Iqbal: Org. Synth. 68, 234 (1990).
C.D. Gutsche and L.-G. Lin: Tetrahedron 42, 1633 (1986).
S.R. Hall, H.D. Flack, and J.M. Stewart (Eds): The XTAL 3.2 Reference Manual, Universities of Western Australia, Geneva and Maryland (1992).
G. Gokel: in J.F. Stoddart (ed.), Crown Ethers and Cryptands, Monographs in Supramolecular Chemistry, No. 3, Royal Society of Chemistry, Cambridge (1991).
C.D. Gutsche: in J.F. Stoddart (ed.), Calixarenes, Monographs in Supramolecular Chemistry, No. 1 Royal Society of Chemistry, Cambridge (1989), p. 108.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Asfari, Z., Nicolle, X., Vicens, J. et al. Ditopic Calixcrowns with Inequivalent Crown Loops: Synthesis and Structural Characterisation of an Unsymmetrical Calix[4]arene biscrown-6. Journal of Inclusion Phenomena 33, 251–262 (1999). https://doi.org/10.1023/A:1008062510582
Issue Date:
DOI: https://doi.org/10.1023/A:1008062510582