Abstract
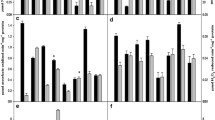
There are several mechanisms used by plants for survival in adverse environments such as drought, high temperature and salinity. The objective of this study was to evaluate the drought tolerance of tepary bean as a function of biochemical processes linked to isozyme synthesis and changes in enzymatic activity related to proline metabolism. Mature seeds of common beans var. flor de mayo, Phaseolus vulgaris and tepary beans Phaseolus acutifolius were grown under two water conditions (irrigation and drought), and four levels of urea. Vertical electrophoresis and spectrophotometric techniques were used to evaluate protein patterns, glutamate dehydrogenase (GDH), proline oxidase (PO) and pyrroline-5-carboxylate reductase (P5C reductase) enzyme activities. These enzymes were studied because they are directly related to protein synthesis. Electrophoretic patterns showed more proteins in tepary beans than in common beans with limited irrigation. GDH showed only one isozyme, with a molecular weight between 240 to 270 kDa. A decrease in PO activity was observed in common beans under drought stress with a value of 237 μmol/min, in comparison to irrigation conditions of 580 μmol/min. GDH and P5C reductase enzymes have had higher activity in common beans than in tepary beans under water stress. There was a significant difference only in glutamate dehydrogenase enzyme with respect to urea level. The results suggest that drought tolerance of tepary beans is due to biochemical processes related to proline metabolic enzymes.
Similar content being viewed by others
References
Deshpande SS (1992) Food legumes in human nutrition: A personal perspective. Crit Rev Food Sci Nutr 32: 333–363.
Koehler HH, Iung-Hsia CH, Sceier G, Burke DW (1987) Nutrition, composition, protein quality and sensory properties of thirty six cultivars of dry beans (Phaseolus vulgaris). J Food Sci 52: 1335–1340.
Meiners CR, Derise NL, Lau HC, Crews MG, Ritchey SJ, Murphy EW (1976) Proximate composition and yield of raw and cooked mature dry legumes. J Agric Food Chem 24: 1122–1126.
Doughty J, Walker A (1982) Etude FAO: Alimentation et Nutrition, FAO, Rome, pp 120.
Salunkhe DK, Kadam SS (1989) CRC Handbook of World Food Legumes: Nutritional Chemistry, Processing Technology and Utilization, Vol 1, Boca Raton: FL CRC Press, pp 495.
Reyes MC, Paredes LO (1993) Hard to cook phenomenon in common beans. A review. Crit Rev Food Sci Nutr 33: 227–286.
Tinsley AM, Cheerens JC, Alegbejo JO, Adan FH, Kruhar KC, Butler LE, Kopplin MJ (1985) Tepay beans. (Phaseolus acutifoliusvar latifolius): a potential food source for African and Middle Eastern cultures. Qual Plant Foods Hum Nutr 35: 87–101.
Waines JG (1978) Protein contents, grain weights, and breeding potential of wild and domesticated tepary beans. Crop Sci 18: 587–589.
Santos DS, Ochoa AN (1990) Adaptación de las plantas al déficit hÍdrico. Ciencia. 41: 333–340.
Bonhert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. The Plant Cell 7: 1099–1111.
Hanson AD, Hitz WD (1982) Metabolic responses of mesophytes to plant water deficits. Ann Rev Plant Physiol 33: 163–182.
Hames D (1990) One dimensional polyacrylamide gel electrophoresis. Analysis of gels following electrophoresis. In: D Hames & D Rickwood. Gel Electrophoresis of Proteins. A Practical Approach 2nd. ed. New York: Oxford University Press, pp 895.
Howere ChK, Splittstoesser WE (1972) Glutamate dehydrogenase from pumpkin cotyledons. Plant Physiol 49: 531–554.
Tanksley SD, Orton TJ (1983) Isozymes in plant genetics and breeding. Part A. Holland Ed Elsevier, pp 473–486.
Huang HC, Cavalieri JA (1979) Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol 63: 531–535.
Sudhakar C, Reddy PS, Veeranjaneyulu K (1993) Effect of salt stress on the enzymes of proline synthesis and oxidation in green gram (Phaseolus aureusRoxb) seedlings. J Plant Physiol 141: 621–623.
Bidwell RGS (1990) FisiologÍa Vegetal. AGT Editor. México, pp 687–692.
Cloutier Y (1983) Changes in the electrophoretic patterns of the soluble proteins of winter wheat and rye following cold acclimation and dessication stress. Plant Physiol 71: 400–403.
Dhindsa RS, Cleland RE (1975) Water stress and protein synthesis. II. Interaction between water stress, hydrostatic pressure, and abscisic acid on the pattern of protein synthesis in avena coleoptiles. Plant Physiol 55: 782–785.
Bensen RJ, Boyer JS, Mullet JE (1988) Water deficit-induced changes in abscisic acid, growth, polysomes, and translatable RNA in soybean hypocotyls. Plant Physiol 88: 289–294.
Pérez E, Balch M, Gidekel M, Segura NM, Herrera EL, Ochoa AN (1996) Effects of water stress on plant growth and root proteins in three cultivars of rice (Oryza sativa) with different levels of drought tolerance. Physiol Plant 96: 284–290.
Loulakakis KA, Kalliopi AR (1996) The seven NAD(H) glutamate dehydrogenase isoenzymes exhibit similar anabolic and catabolic activities. Physiol Plant 96: 29–35.
Loulakakis KA, Kalliopi AR (1992) Ammonium induced increase in NADH-glutamate dehydrogenase activity caused by the de novo synthesis of the alpha subunit. Planta 187: 322–327.
Smith EL, Austen BM, Blumenthal KM, Nyc JF (1975) Glutamate dehydrogenase. In: Boyer PP. The Enzymes, Vol XI Part A, USA: Academic Press, pp 293–367.
Bohnert HJ, Jensen RG (1996) Strategies for engineering water stress tolerance in plants. Trend Biotech 3: 89–97.
Kavi KPB, Hong Z, Miao G, Hu ChA, Verma DS (1995) Overexpression of pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108: 1387–1394.
Delauney JA, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Minireview. The Plant Journal 2: 215–223.
Bogges FS, Stewart RC, Aspinall D, Paleg GL (1976) Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol 58: 398–401.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barrón, M.C., de Mejía, E.G. Comparative study of enzymes related to proline metabolism in tepary bean (Phaseolus acutifolius) and common bean (Phaseolus vulgaris) under drought and irrigated conditions, and various urea concentrations. Plant Foods Hum Nutr 52, 119–132 (1998). https://doi.org/10.1023/A:1008011529258
Issue Date:
DOI: https://doi.org/10.1023/A:1008011529258