Abstract
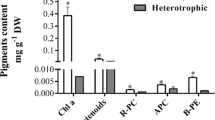
Effects of hydrodynamic stress, dissolved oxygen (DO) concentration and carbon sources on heterotrophic α-tocopherol production by Euglena gracilis were investigated. In a jar fermentor without baffle plates, increasing the agitation speed up to 500 rpm had no significant effect on cell growth and α-tocopherol production. However, in a jar fermentor equipped with baffle plates, both the cell growth and α-tocopherol production were highly suppressed at 500 rpm. At high hydrodynamic stress, the cells secreted nucleic acid-related substances to the culture broth and the shape of the cells shifted from elongated toward spherical. High DO concentration had adverse effects on both cell growth and α-tocopherol production, the optimum DO concentration being below 0.8 ppm. In comparison with glucose, the growth rate was lower but the α-tocopherol content of the cells was almost four times higher when ethanol was used as the organic carbon source. In a fed-batch culture with ethanol, a very high cell concentration of 39.5 g L-1 was obtained with α-tocopherol content of 1200 µg g-cell-1. This α-tocopherol content is very close to the values reported for photoautotrophic and photoheterotrophic cultures. A very high α-tocopherol productivity of 102 µg L-1 h-1 was obtained, indicating that heterotrophic cultivation of E. gracilis has a very high potential as a substitute for the current method of extraction from vegetable oils.
Similar content being viewed by others
References
Al-Rubeai M, Singh RP, Goldman MH, Emery AN (1995) Death mechanisms of animal cells in conditions of intensive agitation. Biotechnol. Bioengng 45: 463–472.
Ben-Shaul Y, Epstein HT, Schiff JA (1965) Studies of chloroplast development in Euglena, 10. The return of the chloroplasts to proplastid condition during dark adaptation. Can. J. Bot. 43: 129–136.
Camara B, Bardat F, Seye A, D'Harlingue A, Monéger R (1982) Terpenoid metabolism in plastids. Plant Physiol. 70: 1562– 1563.
Chen F, Johns MR (1994) Substrate inhibition of Chlamydomonas reinhardtii by acetate in heterotrophic culture. Process Biochem. 29: 245–252.
Chen F, Johns MR (1996) Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochem. 31: 601–604.
Ehara T, Shikira-Ishikawa I, Osafune T, Hase E, Ohkuso I, (1975) Some structural characteristics of chloroplast degeneration in cells of Euglena gracilis Z during their heterotrophic growth in darkness. J. Electron Microscopy 24: 253–261.
Endo H, Hosoya H, Koibuchi T (1977) Growth yields of Chlorella regularis in dark-heterotrophic continuous cultures using acetate. J. Ferment. Technol. 55: 369–379.
Janiszowska W (1987) Intracellular localization of tocopherol biosynthesis in Calendula officinalis. Phytochemistry 26: 1403–1407.
Märkl H, Bronnenmeier R, Wittek B (1991) The resistance of microorganisms to hydrodynamic stress. Int. Chem. Engng. 31: 185–197.
Miyatake, K (1986) Euglena no baiyou (in Japanese), 'Iden'40: 49–54.
Ogbonna JC, Märkl H (1993) Nutrient-split feeding strategy for dialysis cultivation of E. coli. Biotechnol. Bioengng 41: 1092–1100.
Ogbonna JC, Yada H, Tanaka H (1995a) Kinetic study on light-limited batch cultivation of photosynthetic cells. J. Ferment. Bioengng 80: 259–264.
Ogbonna JC, Yada H, Tanaka H (1995b) Light supply coefficient – a new engineering parameter for photobioreactor design. J. Ferment. Bioengng 80: 369–376.
Ogbonna JC, Tanaka H (1996) Night biomass loss and changes in biochemical composition of cells during light/dark cyclic culture of Chlorella pyrenoidosa. J. Ferment. Bioengng. 82: 558–564.
Ogbonna JC, Yada H, Masui H, Tanaka H (1996) A novel internally illuminated stirred tank photobioreactor for large-scale cultivation of photosynthetic cells. J. Ferment. Bioengng 82: 61– 67.
Ogbonna JC, Tanaka H (1997) Industrial-size photobioreactors. Chemtech. 27: 43–49.
Ogbonna JC, Masui H, Tanaka H (1997) Sequential heterotrophic/autotrophic cultivation – an efficient method of producing Chlorella biomass for health food and animal feed. J. appl. Phycol. 9: 359–366.
Reinbothe S, Reinbothe C, Krauspe R, Parthier B (1991) Changing gene expression during dark-induced chloroplast dedifferentiation in Euglena gracilis. Plant Physiol. Biochem. 29: 309–318.
Schwarz T, Bartholmes P, Kaufmann M (1995) Large-scale production of algal biomass for protein purification – tryptophan synthase from Euglena gracilis. Biotechnol. appl. Biochem. 22: 179–190.
Shigeoka S, Onishi T, Nakano Y, Kitaoka S (1986) The contents and subcellular distribution of tocopherols in Euglena gracilis. Agric. biol. Chem. 50: 1063–1065.
Soll J, Kemmerling M, Schultz G (1980) Tocopherol and plastoquinone synthesis in Spinach chloroplasts subfractions. Arch. Biochem. Biophys. 204: 544–550.
Taketomi H, Soda K, Katsui G (1983) Results of screening test in tocopherol in microbial realm. Vitamins (Japan) 57: 133–138.
Takeyama H, Kanamaru A, Yoshino Y, Kakuta H, Kawamura Y, Matsunaga T (1997) Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E by two-step culture of Euglena gracilis Z. Biotechnol. Bioengng 53: 185–190.
Tanaka H (1981) Technological problems in cultivation of plant cells at high density. Biotechnol. Bioengng 23: 1203–1218.
Tanaka H, Takahashi J, Ueda K (1975) A standard for the intensity of agitation shock on mycelia on agitation of mycelia suspension. J. Ferment. Technol. 53: 18–26.
Tani Y, Tsumura H (1989) Screening for tocopherol-producing microorganisms and α-tocopherol production by Euglena gracilis Z. Agric. biol. Chem. 53: 305–312.
Tani Y, Osuka S (1989) α-Tocopherol production by an analog-resistant strain of Euglena gracilis Z. Agric. Biol. Chem. 53: 2313–2318.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ogbonna, J.C., Tomiyamal, S. & Tanaka, H. Heterotrophic cultivation of Euglena gracilis Z for efficient production of α-tocopherol. Journal of Applied Phycology 10, 67–74 (1998). https://doi.org/10.1023/A:1008011201437
Issue Date:
DOI: https://doi.org/10.1023/A:1008011201437